The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately
Express your answer using two significant figures. A approximately what is the normal boiling point and what is the normal melting point of the substance.
 Physical Chemistry Boiling Point Lower Than Melting Point
Physical Chemistry Boiling Point Lower Than Melting Point
The normal boiling point is just one point where the vapourliquid equilibrium line occurs at one atmosphere pressure.
The normal boiling point for the substance in the phase diagram below is approximately. Which point represents an equilibrium between the solid liquid and gas phase. B estimate the normal freezing point of the substance. The phase diagram of a substance is shown below.
Answer the following questions based on the following phase diagram given below. Do u think simon is good fo the show. How do you estimate the normal boiling point of the substance using a phase diagram.
The phase diagram of a hypothetical substance is shown below. Part a water vapor originally at 0005 atm and 05c is slowly compressed at constant temperature until the final pressure is 20 atm. The word normal here refers to 1 atm pressure.
Which line segment represents an equilibrium between fusion and freezing. Estimate the normal boiling point of the substance. Estimate the normal freezing point of the substance.
If you can read graphs this should be easy. The approximate normal boiling point of this substance is 300 k imagine a reaction that results in a change in both volume and temperature as shown in the diagram below. How do you estimate the normal boiling point of the substance using a phase diagram.
Express your answer using two significant figures. Express your answer using two significant figures. Refer to the figure and describe all the phase changes that would occur in each of the following cases.
For a certain substance the normal melting point is 60oc the normal boiling point is. Approximately what is the normal melting point of the substance. For the mp id read approximately 180k and for the nbp about 300k.
T 150 k. Tell me who u want to win. In the generic phase diagram shown below you can.
What is the pressure of the substance at the triple. At what temperature can we no longer tell the difference between the liquid and the gas. The phase diagram of a substance is shown below.
Express your answer using two significant figures. What is the physical state of the substance under the following conditions. A phase diagram is a lot of points at differing temperatures and pressures.
Figure 1 a estimate the normal boiling point of the substance. If u watch american idol give me a star. What is the temperature of the substance at the triple point.
Approximately what is the normal boiling point. C what is the physical state of the substance under t 150 k p.
 What Is The Meaning Of Triple Point Of Water Quora
What Is The Meaning Of Triple Point Of Water Quora
 Solved Phase Diagram Worksheet A What Is The Normal Melt
Solved Phase Diagram Worksheet A What Is The Normal Melt
 Solved The Phase Diagram Of A Substance Is Shown Below A
Solved The Phase Diagram Of A Substance Is Shown Below A
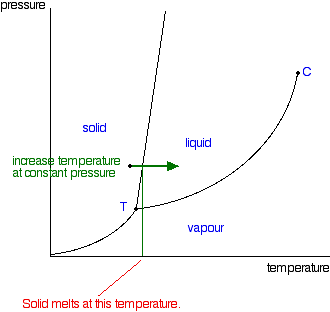 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
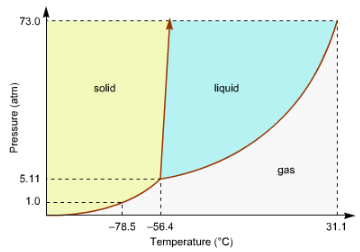 Consider This Phase Diagram For Carbon Dio Clutch Prep
Consider This Phase Diagram For Carbon Dio Clutch Prep
Chemistry The Central Science Chapter 11 Section 6
Quick Review Use The Phase Diagram Below To Answer The
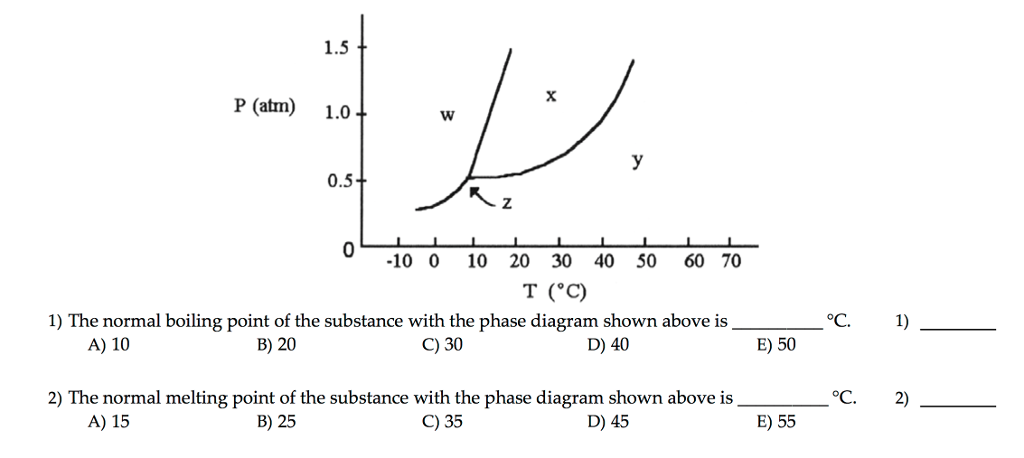 Solved The Normal Boiling Point Of The Substance With The
Solved The Normal Boiling Point Of The Substance With The
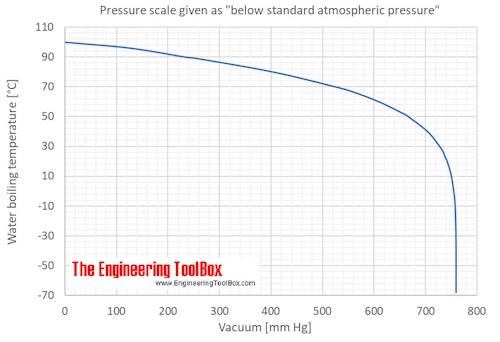 Water Boiling Points At Vacuum Pressure
Water Boiling Points At Vacuum Pressure
 Phase Diagrams Critical Point Triple Point And Phase
Phase Diagrams Critical Point Triple Point And Phase
 Intermolecular Forces Interpreting A Phase Diagram
Intermolecular Forces Interpreting A Phase Diagram
 Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
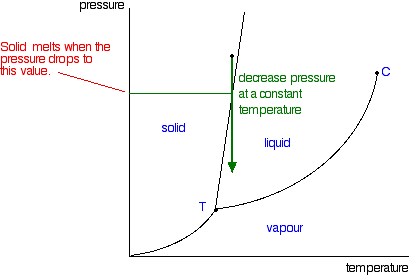 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Boiling Point From Pvt Diagram Example Youtube
Boiling Point From Pvt Diagram Example Youtube
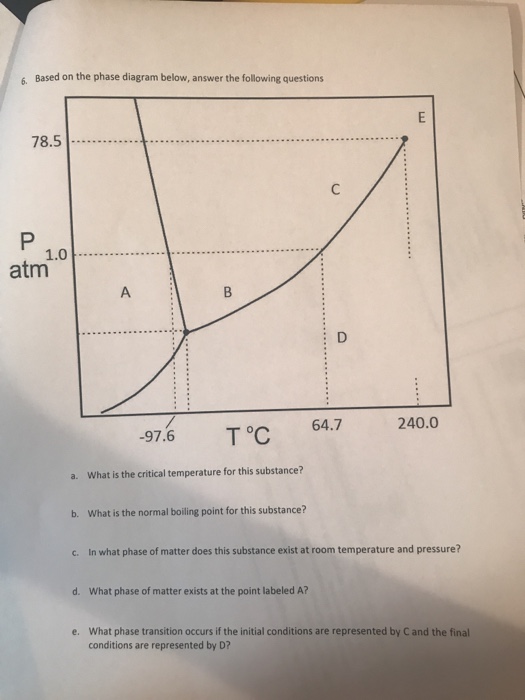
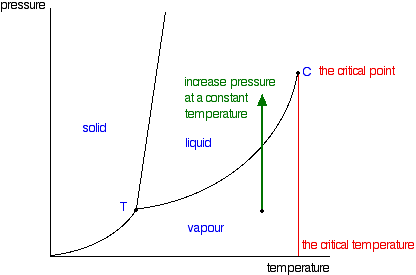 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
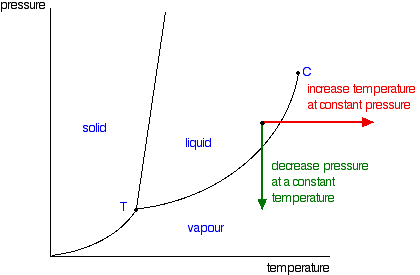 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 The Normal Boiling Point Of The Substance With The Phase
The Normal Boiling Point Of The Substance With The Phase
 Answer The Following Questions Based On Th Clutch Prep
Answer The Following Questions Based On Th Clutch Prep
 Sublimation Of Iodine Rise And Fall Of A Misconception
Sublimation Of Iodine Rise And Fall Of A Misconception
Chemistry The Central Science Chapter 11 Section 6
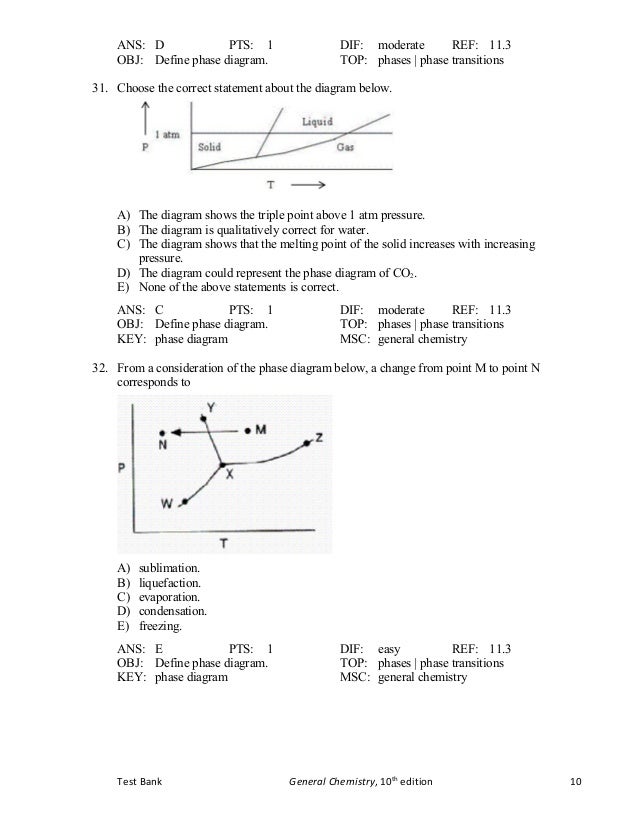 Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
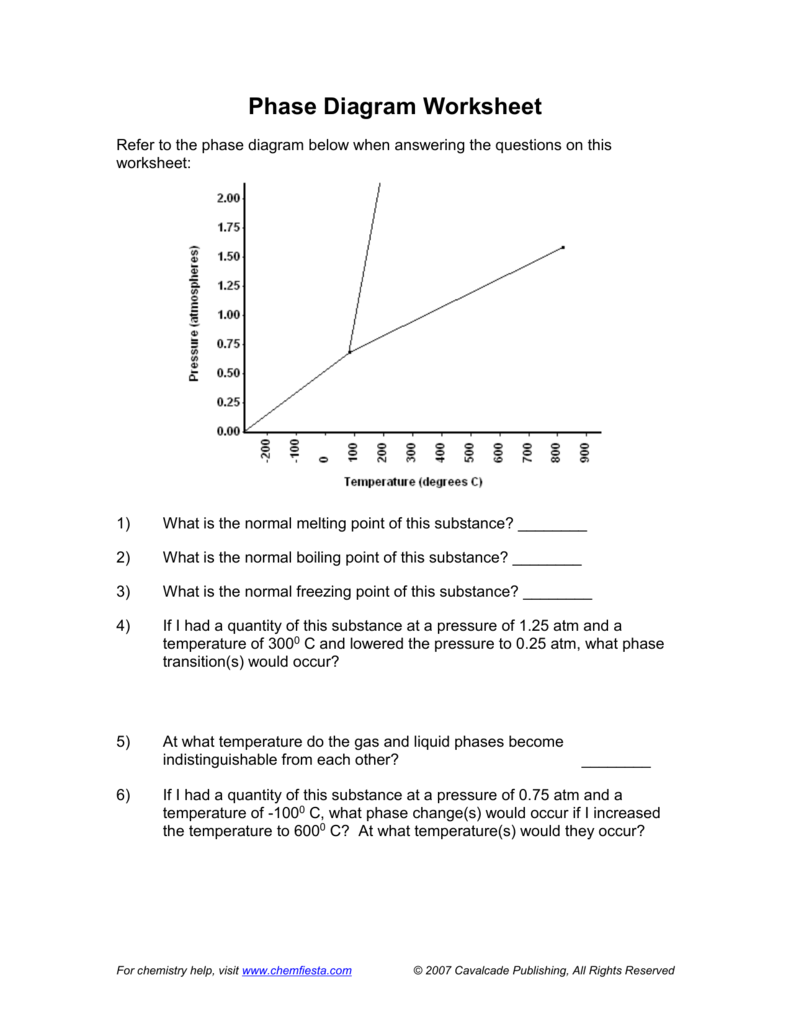
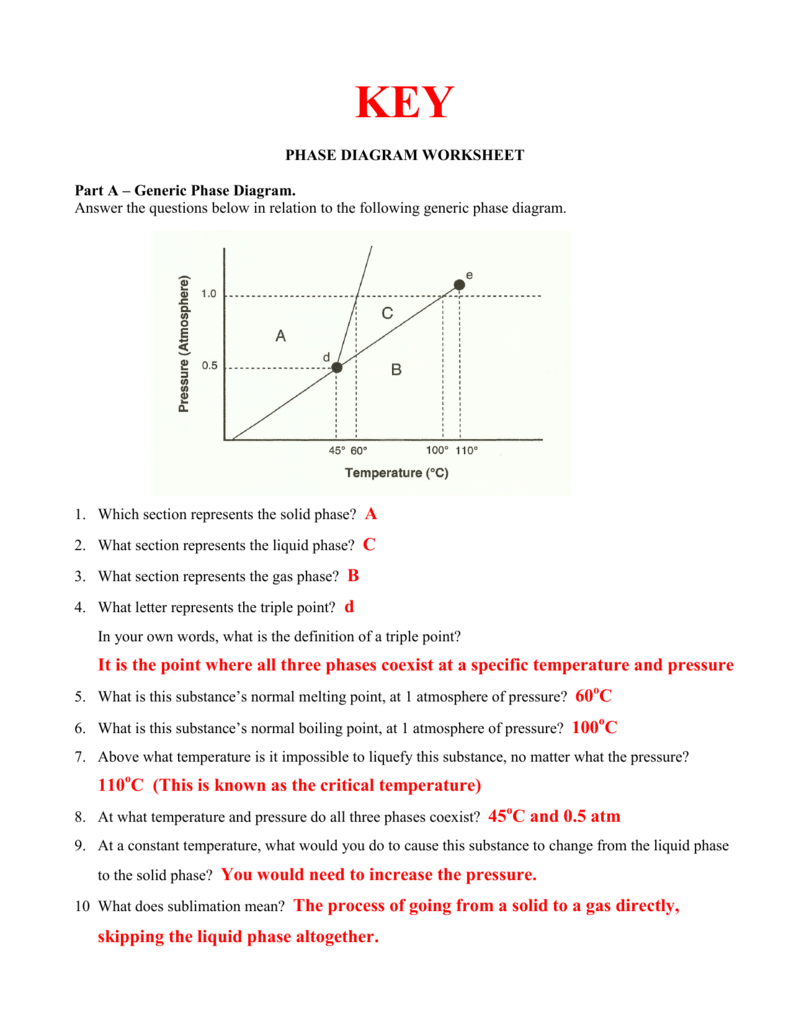
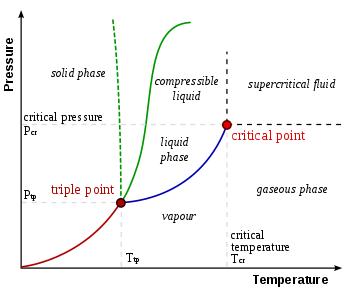


0 Response to "The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately"
Post a Comment