Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System
I think you might increase the energy if you decrease the volume under pressure because the temperature will increase and you will have done work on the system hence increasing it internal energy. A closed nonreactive system contains species 1 and 2 in vaporliquid equilibrium.
Renewable Energy Our World In Data
Some additional moles of species 2 are added to the system which is then restored to its.

Does this diagram represent an increase or decrease in the internal energy of the system. A a system absorbs 105 kj of heat from its surroundings while doing 29 kj of work on the surroundings. Use the order calculator below and get started. A does this diagram represent an increase or decrease in the internal energy of the system.
Species 2 is a very light gas essentially insoluble in the liquid phase. Temperature increase or decrease. Consider the accompanying energy diagram.
C the system releases 575 kj of heat while doing 225 kj of work on the surroundings. Does this diagram represent an increase or decrease in the internal energy of the system. Contact our live support team for any assistance or inquiry.
B the internal energy of a system decreases when work is done on the system and heat is flowing into the system c the system does work on the surroundings when an ideal gas expands against a constant external pressure. It may increase or decrease whether the process is reversible or irreversible is irrelevant because that may be determined by the other terms such as internal energy state u and pressure volume pv. When heat flows to or from a system the system gains or loses an amount of energy equal to the amount of heat transferred heat added to a system increase in internal energy external work done by the system.
A does this diagram represent an increase or decrease in the internal energy of the system. The vapor phase contains both species 1 and 2. B q 150 kj and w 657 j.
It represents a component of the gibbs free energy of that system. The area under a ts diagram has units of energy. Answer to visualizing conceptsconsider the accompanying energy diagram a does this diagram represent an increase or decrease in.
Calculate delta e energy and determine whether the process is endothermic or exothermic for the follwing cases. In thermodynamics thermal energy is the internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. In a loose sense thermal energy is a term often used to describe the energy content of a system related to heating effects eg.
A the internal energy of a system increases when more work is done by the system than heat was flowing into the system.
 Thermodynamic Process An Overview Sciencedirect Topics
Thermodynamic Process An Overview Sciencedirect Topics
The First Law Of Thermodynamics Closed Systems Heat Transfer
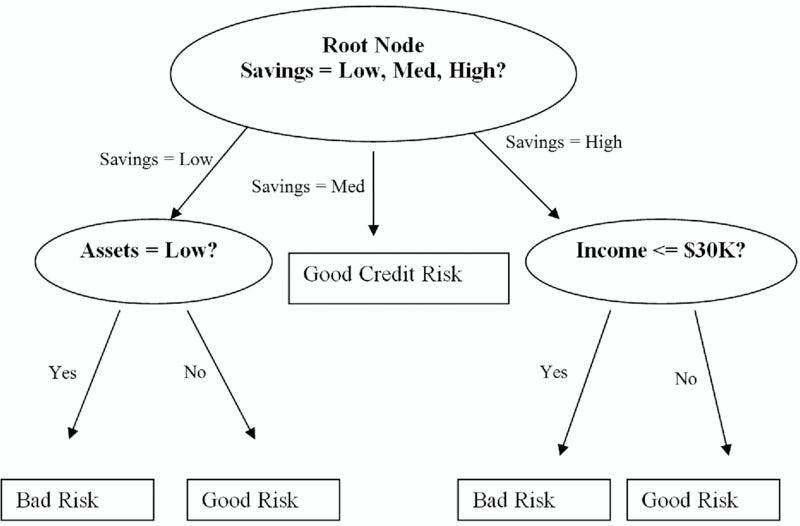 Decision Trees A Simple Way To Visualize A Decision
Decision Trees A Simple Way To Visualize A Decision
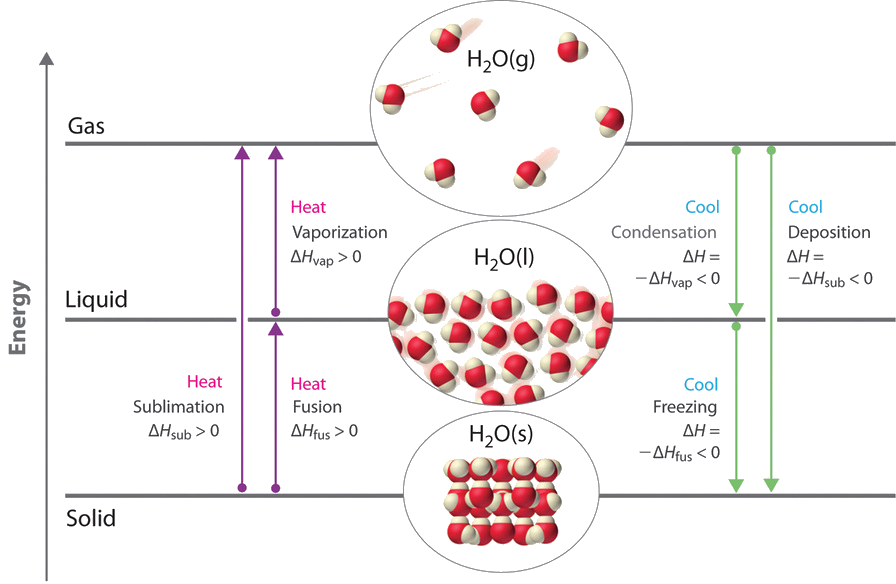 11 4 Phase Changes Chemistry Libretexts
11 4 Phase Changes Chemistry Libretexts
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png) Endothermic And Exothermic Chemical Reactions
Endothermic And Exothermic Chemical Reactions

 The Three Laws Of Thermodynamics Eagle Blog
The Three Laws Of Thermodynamics Eagle Blog
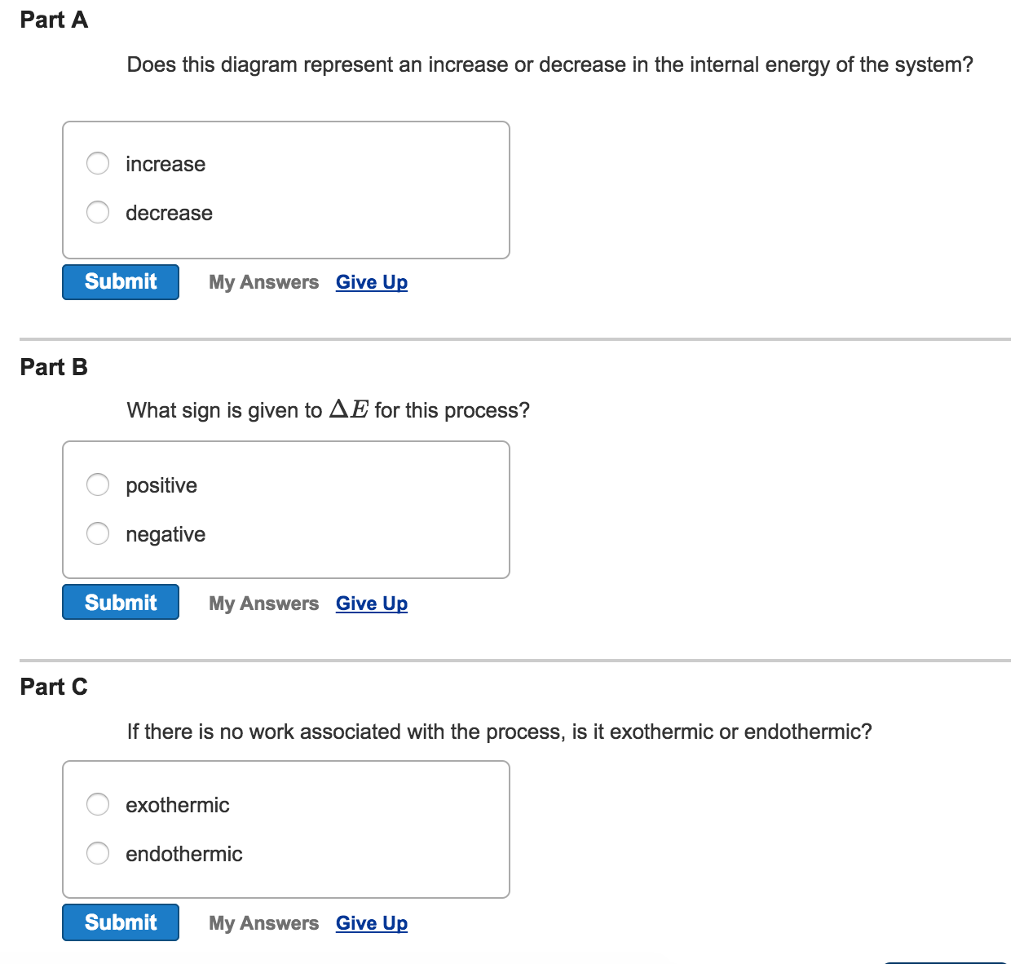 Solved Consider The Energy Diagram Shown In The Figure F
Solved Consider The Energy Diagram Shown In The Figure F
 The First Law Of Thermodynamics Was Given As Q Deltau W
The First Law Of Thermodynamics Was Given As Q Deltau W
System Behavior And Causal Loop Diagrams
 Chapter 3a The First Law Closed Systems Energy
Chapter 3a The First Law Closed Systems Energy
 Metabolism Definition Process Biology Britannica Com
Metabolism Definition Process Biology Britannica Com
 Figure Title Potential Energy And Kinetic Energy Caption
Figure Title Potential Energy And Kinetic Energy Caption
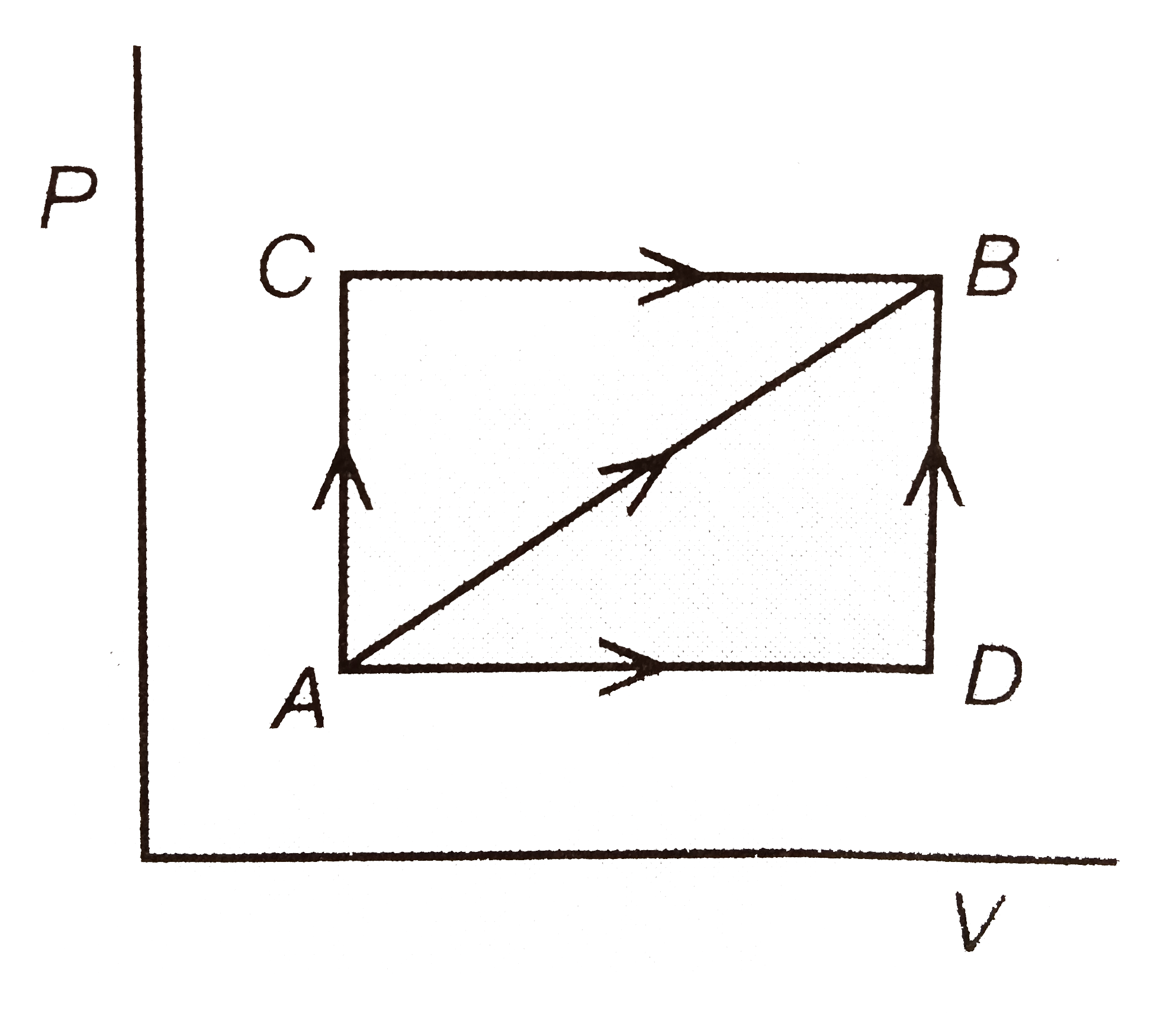
 Pressure Volume Diagram Energy Education
Pressure Volume Diagram Energy Education
 Energy Enthalpy And The First Law Of Thermodynamics
Energy Enthalpy And The First Law Of Thermodynamics
Hertzsprung Russell Diagram Cosmos
Chemistry The Central Science Chapter 5 Section 2
 What S The Difference Between Ac Induction Permanent Magnet
What S The Difference Between Ac Induction Permanent Magnet


0 Response to "Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System"
Post a Comment