Phase Change Diagram For Rubbing Alcohol
Before rubbing alcohol absorbs it is a liquid. 5087 k 2356 c 5370 kpa std enthalpy change of fusion δ fus h o.
Isopropyl Alcohol Organic Method 109
With water and ice in a closed thermos bottle both the liquid phase and solid phase of water exist at the same time in the same place.
Phase change diagram for rubbing alcohol. In order for a substance to move between the states of matter. The attractive forces between the liquid alcohol molecules are weaker than the attractive forces between liquid water molecules. It is prepared from a special denatured alcohol solution and contains 975 100 by volume of pure concentrated ethonal.
Example of a general phase diagram. 1 with the y axis representing increasing temperature and the x axis sample heating time. Change the units of pressure and temperature to atm and c respectively by clicking on each window and selecting change units figure 6.
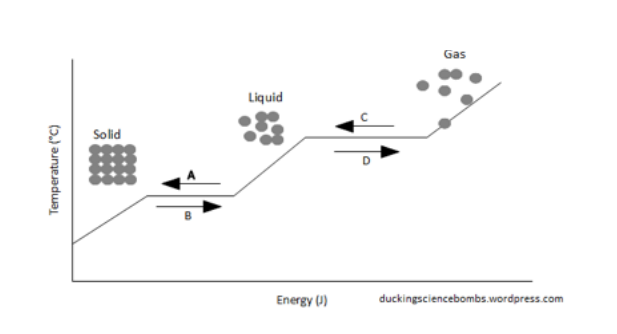
440 kjmol std entropy change of vaporization δ vap s o. 2 of the labquest2. For example to turn from a solid into a liquid which is called fusion or from a gas to a liquid vaporization energy must be gained or lost.
1 and temperature probe into ch. Chromatography is a process for separating different components from a mixture. Phase behavior triple point.
528 kjmol std entropy change of fusion δ fus s o. As the alcohol takes in heat it turns into a gas. Isopropyl alcohol cas 67 63 0 chemical physical properties by before rubbing alcohol absorbs it is a liquid.
12716 5 figure 4. While it absorbs heat the phase change occurring is evaporation. Crystalline solid is the temperature at which the forces holding its crystal lattice together are broken and become a liquid.
Phase change diagram for rubbing alcohol. Plug the pressure sensor into ch. Rubbing alcohol is a liquid prepared and used primarily for application.
At higher altitudes the air pressure is lower. Once this phase change is complete additional energy heats the vapor to a final temperature. 1849 k 882 c.
Point x represents the critical point and at the critical point and beyond the substance is forever in the vapor phase. 124 jmolk solid properties std enthalpy change. In doing this students can begin to see patterns between the change in molecular motion and whether a reaction is endothermic or exothermic.
This diagram allows us to explain strange phenomena such as why water boils at a lower temperature at higher altitudes for example. 286 jmolk std enthalpy change of vaporization δ vap h o. They begin to see that if a phase change causes molecules to move faster after the change that it is endothermic and that if the molecules slow down after the phase change it is exothermic.
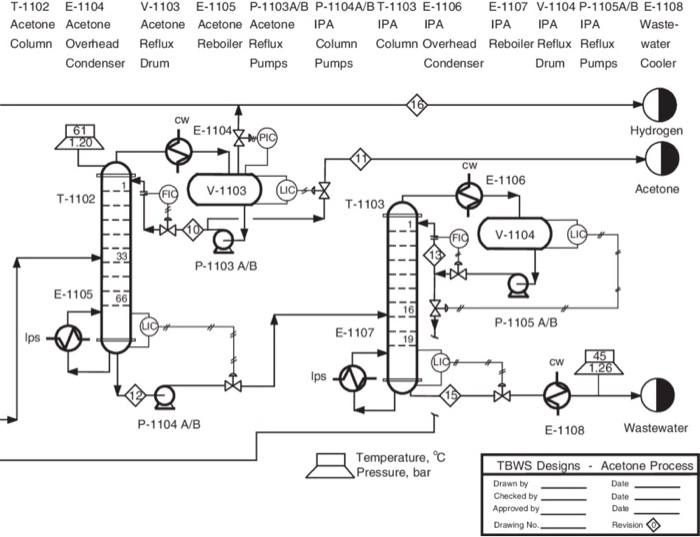 Acetone Is Produced Via Catalytic Dehydrogenation
Acetone Is Produced Via Catalytic Dehydrogenation
 Ethanol Dynamic And Kinematic Viscosity
Ethanol Dynamic And Kinematic Viscosity
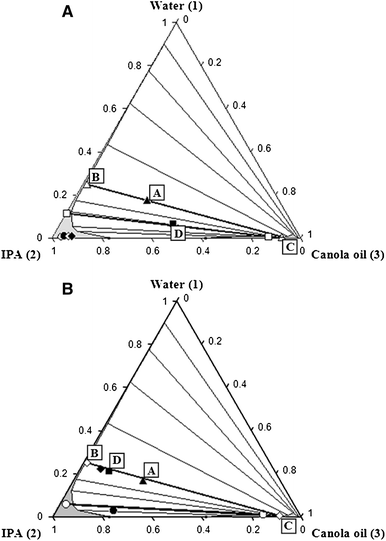 Application Of A Ternary Phase Diagram To The Phase
Application Of A Ternary Phase Diagram To The Phase
Isopropyl Alcohol Cas 67 63 0 Chemical Physical
 Notes 11 3 Obj 11 5 Rubbing Alcohol Feels Cold When It
Notes 11 3 Obj 11 5 Rubbing Alcohol Feels Cold When It
 Skin Antisepsis With Chlorhexidine Alcohol Versus Povidone
Skin Antisepsis With Chlorhexidine Alcohol Versus Povidone
 Phase Changes Substances Can Change From One Phase To
Phase Changes Substances Can Change From One Phase To
The Energy Of Evaporation A Lab Investigation
Solids And Liquids Jeopardy Review Game Answer Key
Dmso As An Entrainer To Separate A Water Isopropyl Alcohol
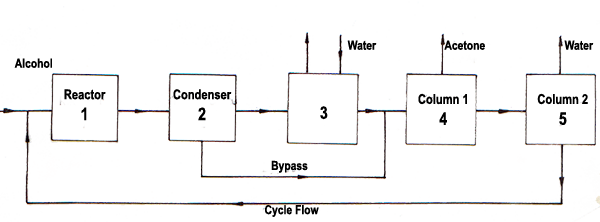 Acetone Production Process From Iso Propyl Alcohol Steemit
Acetone Production Process From Iso Propyl Alcohol Steemit
/phase-changes-56a12ddd3df78cf772682e07.png) List Of Phase Changes Between States Of Matter
List Of Phase Changes Between States Of Matter
 Answer Label Each Letter By Its Phase Dia Clutch Prep
Answer Label Each Letter By Its Phase Dia Clutch Prep
Isopropyl Alcohol Cas 67 63 0 Chemical Physical
Isopropyl Alcohol Organic Method 109
 Shear Viscosity Of Polymer Solutions Of Peo In 95 Water 5
Shear Viscosity Of Polymer Solutions Of Peo In 95 Water 5
 Viscosity Is An Important Factor Of Resistance To Alcohol
Viscosity Is An Important Factor Of Resistance To Alcohol
 Warfarin Sodium Contains Isopropyl Alcohol 98 0 Tci
Warfarin Sodium Contains Isopropyl Alcohol 98 0 Tci
 Ternary Phase Diagrams Of Isopropyl Myristate Ipm
Ternary Phase Diagrams Of Isopropyl Myristate Ipm
Acetone Heat Exchanger Optimisation Process Diagram
0 Response to "Phase Change Diagram For Rubbing Alcohol"
Post a Comment