C2 Molecular Orbital Diagram
Fill from the bottom up with 8 electrons total. The answer is c2 because of bond orders when we draw the c2 mo we have everything up till the pipy orbitlal filled and the next orbital tht would be filled would be the sigma2pz orbital.
 Why Both The Bonds In C2 Molecule Are Pi Bonds Quora
Why Both The Bonds In C2 Molecule Are Pi Bonds Quora
The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.

C2 molecular orbital diagram. Bonding order is 2 and it is diamagnetic. A mo is defined as the combination of atomic orbitals. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of.
In vb the bond picture arises from considering that the c atom bears a sp hybridization. H 2 n 2 o 2 and f 2. Molecular orbitals the region an electron is most likely to be found in a molecule.
Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined. Summary mo theory lcao mo theory is a simple method for predicting the approximate electronic structure of molecules.
Molecular orbital diagram for carbon dimer c2. Homonuclear diatomics molecules consisting of two identical atoms are said to be homonuclear diatomic such as. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the filling of the molecular orbitals by electrons. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. The formal bond order calculated with these orbitals and occupation numbers is 2 resulting from 6 electrons in bonding orbitals and 2 in an antibonding orbital.
As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. Next well see that symmetry will help us treat larger molecules in. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
Schematic picture of the molecular orbital diagram obtained from mo theory.
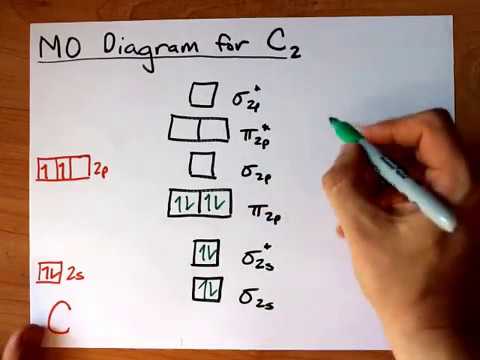 Molecular Orbital Mo Diagram Of C2
Molecular Orbital Mo Diagram Of C2

 What Is The Molecular Orbital Diagram For O2 And O2 Ions
What Is The Molecular Orbital Diagram For O2 And O2 Ions
 Diagrama De Orbitales Moleculares Del Carbono C2
Diagrama De Orbitales Moleculares Del Carbono C2
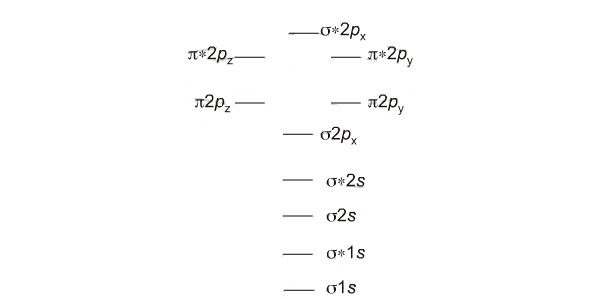 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
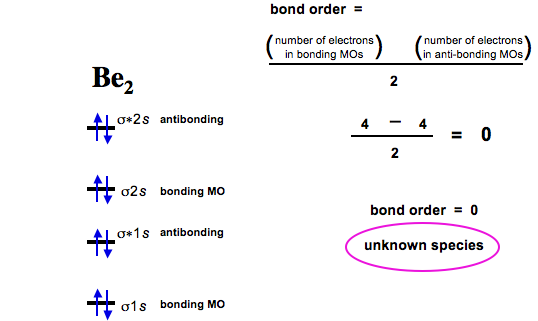 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 How Would The Bond Lengths Vary In Di Carbon Species C2 C2
How Would The Bond Lengths Vary In Di Carbon Species C2 C2

 Diatomic Molecular Orbital Energy Diagram Chemistry Yahoo
Diatomic Molecular Orbital Energy Diagram Chemistry Yahoo
 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength Magnetic Properties
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength Magnetic Properties
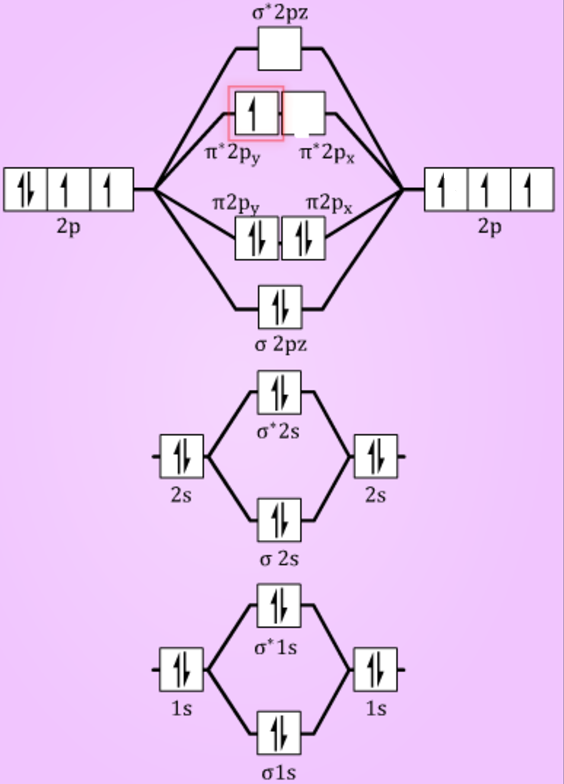 Energy Level Diagram For Molecular Orbitals Chemical
Energy Level Diagram For Molecular Orbitals Chemical
 What Is The Bond Order Of Cl 2
What Is The Bond Order Of Cl 2
 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
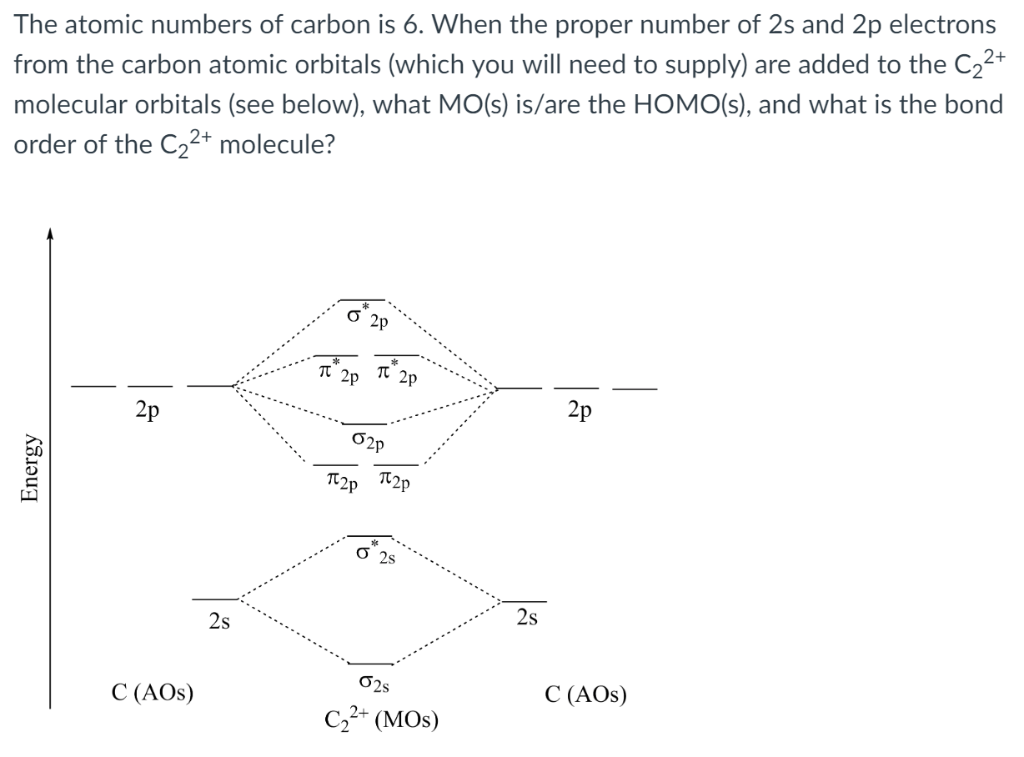 Solved The Atomic Numbers Of Carbon Is 6 When The Proper
Solved The Atomic Numbers Of Carbon Is 6 When The Proper
 What Is The Molecular Orbital Diagram For C 2 Socratic
What Is The Molecular Orbital Diagram For C 2 Socratic
 Why Both The Bonds In C2 Molecule Are Pi Bonds Quora
Why Both The Bonds In C2 Molecule Are Pi Bonds Quora
 A Sp B Sp2 C Sp3 D Sp3d E Sp3d2 Molecular Orbital Theory
A Sp B Sp2 C Sp3 D Sp3d E Sp3d2 Molecular Orbital Theory
Mo Diagrams For Diatomic Molecules


0 Response to "C2 Molecular Orbital Diagram"
Post a Comment