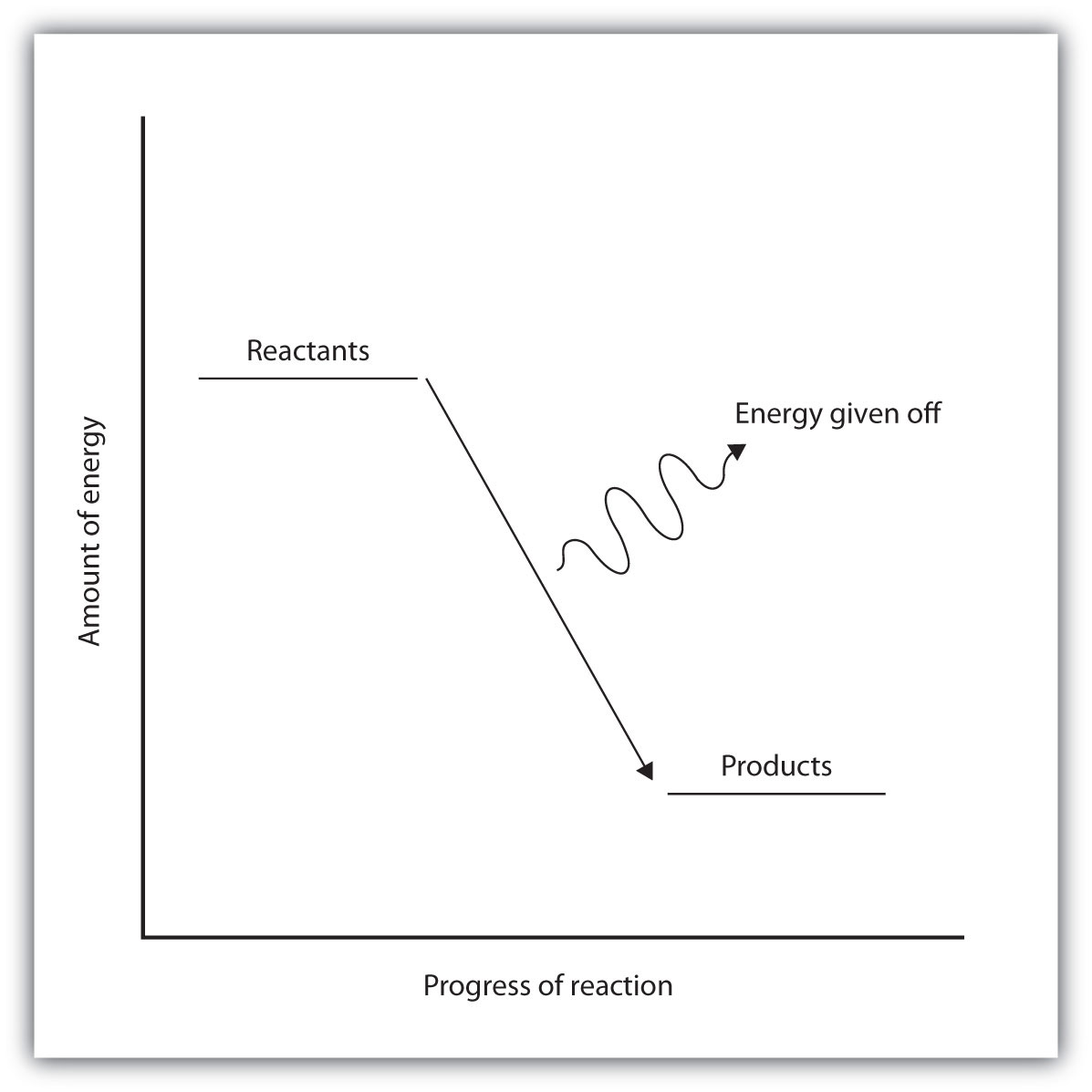
Energy Diagram For Exothermic Reaction
The total potential energy of the system decreases for the exothermic reaction as the system releases energy to the surroundings. In other words the products are less stable than the reactants.
Energy profile diagrams for endothermic and exothermic reactions.

Energy diagram for exothermic reaction. For a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single energetic pathway along the reaction coordinate as the reactants are transformed into products. Energy diagrams for endothermic and exothermic reactions. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction.
This first video takes you through all the basic parts of the pe diagram. The green arrow is longer. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
The amount of heat energy released or absorbed during a chemical reaction is called the heat of reaction. Reaction coordinate diagrams are derived from the corresponding potential energy surface pes which are used in computational chemistry to model chemical reactions by. Energy diagram for an exothermic reaction http academics tctc edu science chm energy profile diagrams endothermic exothermic reactions 4 energy profile diagrams endothermic exothermic reactions 2 energy profiles for chemical reactions.
How to make a graph of endothermic reactions quora. δh total energy content of products total energy content of reactants h products h. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds.
Endothermic and exothermic reactions paul andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. There is a greater difference in energy between the reactants and products. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
The reaction shown by the second diagram is more exothermic. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Heat of reaction is given the symbol ah and is usually measured in kilojoules kj.
It also shows the effect of a catalyst on the forward and reverse activation energy. A in an endothermic reaction the energy of the products is greater than the energy of the. Sometimes a teacher finds it necessary to ask questions about pe diagrams that involve actual potential energy values.
 How To Draw Label Enthalpy Diagrams Video Lesson
How To Draw Label Enthalpy Diagrams Video Lesson
Er10 Temperature Rate And Potential Energy Diagrams
 Kinetics And Equilibrium Ppt Download
Kinetics And Equilibrium Ppt Download
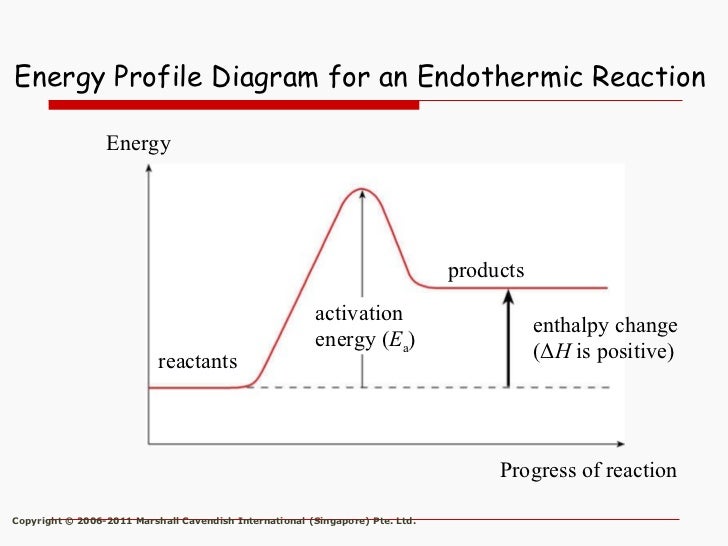 Endothermic Energy Level Diagram
Endothermic Energy Level Diagram
 Endothermic Reaction Detailed Explanation With Examples
Endothermic Reaction Detailed Explanation With Examples
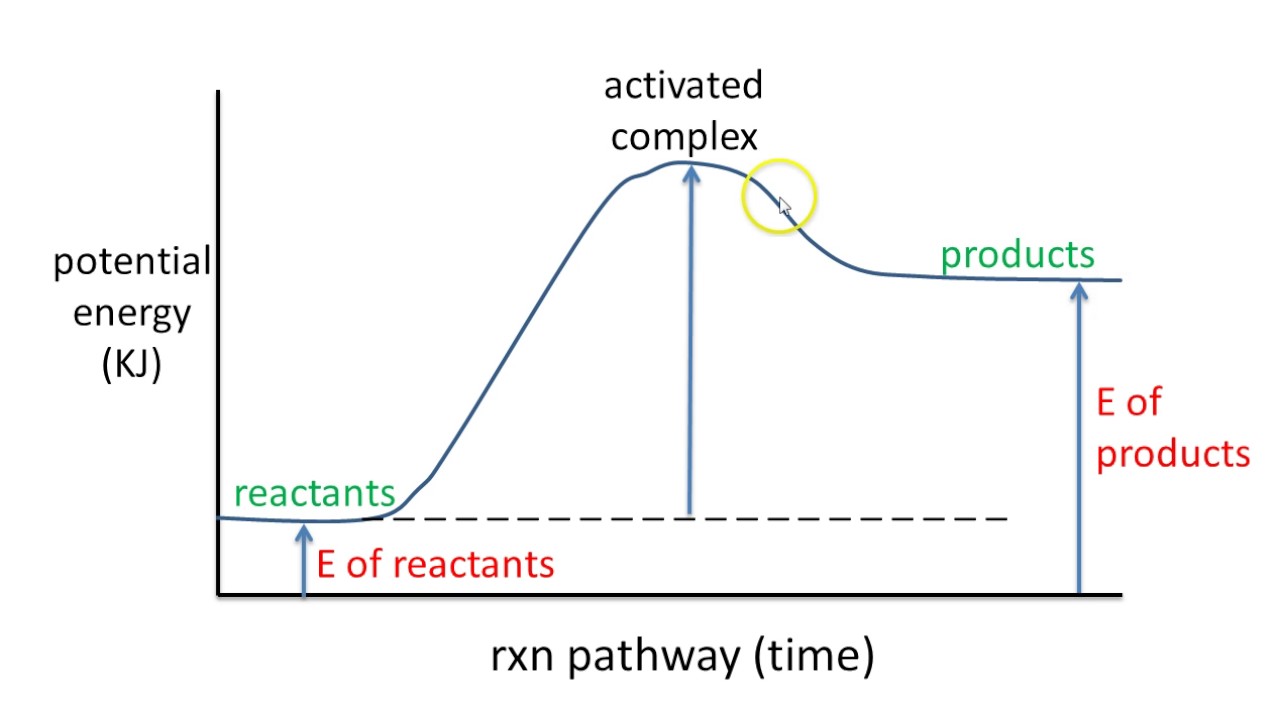 6 Potential Energy Diagram Endothermic Rxn
6 Potential Energy Diagram Endothermic Rxn
Reaction Energy Profiles Activation Energy Exothermic
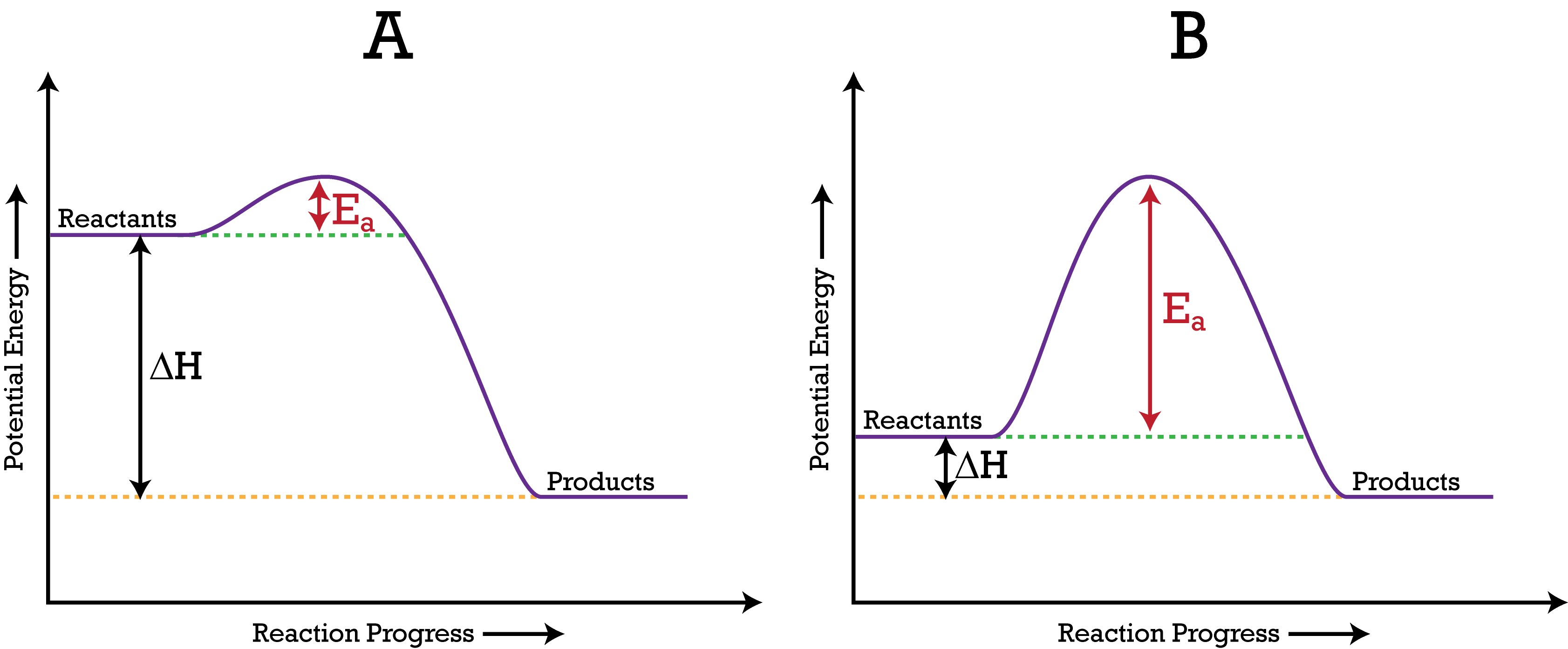
 How To Make A Graph Of Endothermic Reactions Quora
How To Make A Graph Of Endothermic Reactions Quora
 Potential Energy Diagram With Without Catalyst In A
Potential Energy Diagram With Without Catalyst In A
Radical Substitution Reactions Chemgapedia
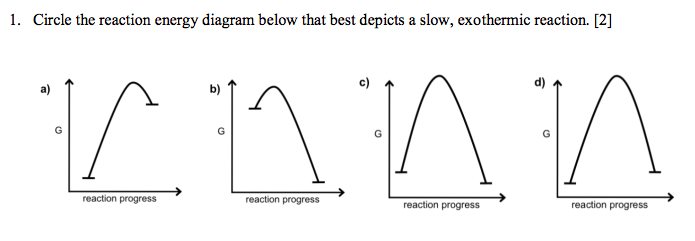 Solved 1 Circle The Reaction Energy Diagram Below That B
Solved 1 Circle The Reaction Energy Diagram Below That B
 4 14 Represent Exothermic And Endothermic Reactions On A
4 14 Represent Exothermic And Endothermic Reactions On A
 How Does The Energy Level Diagram Show This Reaction Is
How Does The Energy Level Diagram Show This Reaction Is
 Endothermic Potential Energy Diagram General Chemistry
Endothermic Potential Energy Diagram General Chemistry
 Which Statement Accurately Describes This Graph It
Which Statement Accurately Describes This Graph It
 Bond Energies And Chemical Reactions
Bond Energies And Chemical Reactions
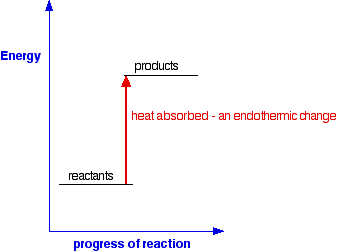 Chemical Energetics An Introduction
Chemical Energetics An Introduction
 What Is Difference Between Endothermic And Exothermic
What Is Difference Between Endothermic And Exothermic
 Exothermic Potential Energy Diagram General Chemistry
Exothermic Potential Energy Diagram General Chemistry


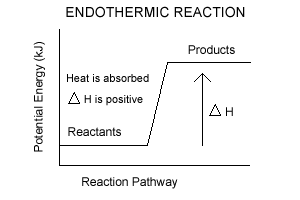
0 Response to "Energy Diagram For Exothermic Reaction"
Post a Comment