Consider The Sugar Water Phase Diagram
B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid. 96 at a pressure of 01 atm determine a the melting temperature for ice and b the boiling temperature for water.
B if the saturated liquid solution in part a is cooled to 20 c 68 f some of the sugar will precipitate out as a solid.
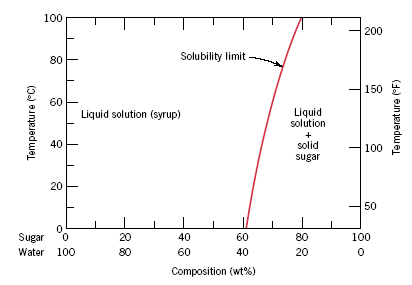
Consider the sugar water phase diagram. On the other hand the boiling temperature is at the intersection of the horizontal line with the liquid vapor. L α adapted from fig. Phase diagrams problem solutions solubility limit 91 consider the sugarwater phase diagram of figure 91.
One component or unary phase diagrams 95 consider a specimen of ice that is at 15c and 10 atm pressure. Phase diagram phase diagrams. That has a distinctly different composition.
Consider the sugarwater phase diagram of figure 101. Consider the sugarwater phase diagram of figure. Horizontally across the pressure temperature diagram at this pressure.
B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar precipitates as a solid. Using figure 92 the pressuretemperature phase diagram for determine the pressure to which the specimen must be raised or lowered to cause it a to melt and b to sublime. A how much sugar will dissolve in 1500 g of water at 90c 194f.
A how much sugar will dissolve in 1500 g water at 90c 194f. A how much sugar will dissolve in 1000 g of water at 90. To illustrate this concept consider the sugarwater c 12h 22o 11h 2o system.
A how much sugar will dissolve in 1500 g water at 90 c 194 f. Initially as sugar is added to water a sugar water solution or syrup forms. The temperature corresponding to the intersection of the ice liquid phase boundary is the melting temperature which is approximately 1c.
A how much sugar will dissolve in 1000 g of water at 80c 176f. Consider the sugarwater phase diagram of figure 91. And types of phases rule 1.
What will be the composition of the saturated liquid solution in wt sugar at 20c. 93a is adapted from phase diagrams of binary nickel alloys p. Consider a sugar water phase diagram.
B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid. As more sugar is introduced the solution becomes more concentrated until the solubility limit is reached or the solution becomes. Using figure 92 the pressuretemperature phase diagram for h 2 o determine the pressure to which the specimen must be raised or lowered to cause it a to melt and b to sublime.
If we know t and co then we know the and types of phases present.
/eutectic-system-phase-diagram-56a135273df78cf7726863ea.png) Eutectic Definition And Examples
Eutectic Definition And Examples
 Phase Diagram Challenge Pdf Name Mece 2340 Challenge Phase
Phase Diagram Challenge Pdf Name Mece 2340 Challenge Phase
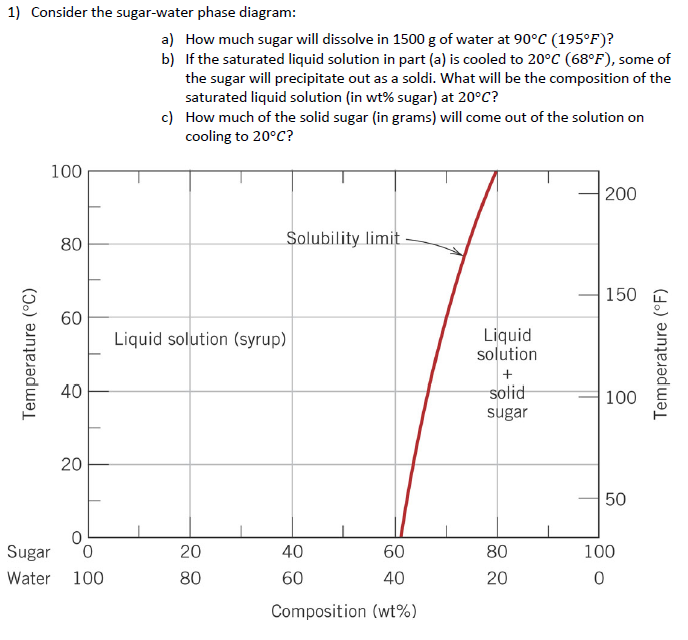 Consider The Sugar Water Phase Diagram How Much S
Consider The Sugar Water Phase Diagram How Much S
Chapter 9 Phase Diagrams Florida International University
 Cooling Curve An Overview Sciencedirect Topics
Cooling Curve An Overview Sciencedirect Topics
 Solved Consider The Sugar Water Phase Diagram Of Figure
Solved Consider The Sugar Water Phase Diagram Of Figure
 Chapter 8 Phase Diagram 1 Phase Diagrams Prof Khaled M
Chapter 8 Phase Diagram 1 Phase Diagrams Prof Khaled M
 Lecture 16 03292018 Phase Diagrams Pptx Phase Diagrams
Lecture 16 03292018 Phase Diagrams Pptx Phase Diagrams
 Chapter 9 Phase Diagrams Materials Science And
Chapter 9 Phase Diagrams Materials Science And
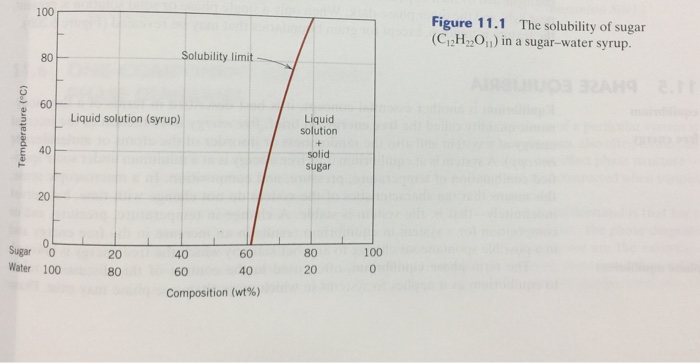
Lecture Notes 8 Hz 2 234 Mat E202 A Materials Science Ii
 Chapter 9 Sections 9 2 9 3 9 4 Ppt Video Online Download
Chapter 9 Sections 9 2 9 3 9 4 Ppt Video Online Download
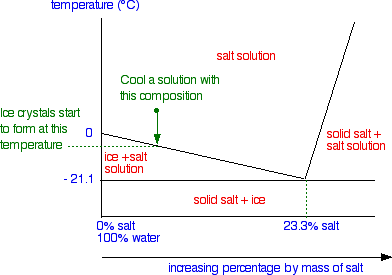 Solid Liquid Phase Diagrams Salt Solution
Solid Liquid Phase Diagrams Salt Solution
 Kcl H2o Phase Diagram Wiring Diagram Directory
Kcl H2o Phase Diagram Wiring Diagram Directory
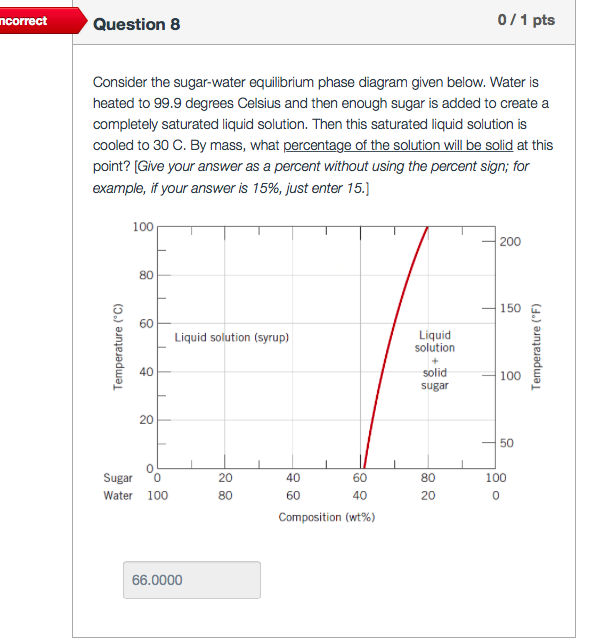 Solved Consider The Sugar Water Equilibrium Phase Diagram
Solved Consider The Sugar Water Equilibrium Phase Diagram
 Diagram Of Sugar Catalogue Of Schemas
Diagram Of Sugar Catalogue Of Schemas
 Solved Consider The Sugar Water Phase Diagram 1 How Mu
Solved Consider The Sugar Water Phase Diagram 1 How Mu
:max_bytes(150000):strip_icc()/eutectic-system-phase-diagram-56a135273df78cf7726863ea.png) Eutectic Definition And Examples
Eutectic Definition And Examples
 Phase Diagrams Chapter Ppt Video Online Download
Phase Diagrams Chapter Ppt Video Online Download

0 Response to "Consider The Sugar Water Phase Diagram"
Post a Comment