Molecular Orbital Diagram For He2 2
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. Construct the molecular orbital diagram for he2 and then identify the bond order.
 Fsc Chemistry Book1 Ch 6 Lec 24 Molecular Orbital
Fsc Chemistry Book1 Ch 6 Lec 24 Molecular Orbital
And total number of electrons available are 4.
Molecular orbital diagram for he2 2. Molecular orbital diagram for carbon dimer c2. This mix to form a sigma orbital from h1sli2s a sigma orbital and h1s li2s and a non bonding orbital from li1s lower in energy than the sigma. Please note the diagram is for he2 but the he h is very similar.
Chemical bonding chemical bonding molecular orbitals of h2 and he2. Its molecular orbitals are constructed from the valence shell orbitals of each hydrogen atom which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference.
Determine the total number of valence electrons in the he 2 2 ion. Li has 1s 2s while h has 1s. In he2 molecule atomic orbitals available for making molecular orbitals are 1s from each helium.
Bonding order is 2 and it is diamagnetic. Molecular orbital energy level diagram bond order and stability. Get more help from chegg.
Fill from the bottom up with 8 electrons total. Electronic configuration molecular behaviour. To enroll in courses follow best educators interact with the community and track your progress.
You fill these molecular orbitals with the electrons as required and then you can. Combine the two he valence atomic orbitals to produce bonding and antibonding molecular orbitals. Get 11 help now from expert chemistry tutors.
Energy level diagram for molecular orbitals. Nonbonding sigma is occupied and then the sigma orbital is occupied. Molecular orbital theory mot presented by megha khandelwal.
In he2 dihelium the two 1s atomic orbitals overlap to create two molecular orbitals. Draw the molecular orbital energy level diagram for the system. The procedure can be introduced by considering the h2 molecule.
Mo theory explains why he2 molecule does not exists. Click within the blue boxes to add electrons. Molecular orbitals thus formed are1s21s2 it means 2 electrons are in bonding molecular orbitals an.
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Fill the molecular orbitals.
 5 7 Molecular Orbital Theory General College Chemistry I
5 7 Molecular Orbital Theory General College Chemistry I
 Videos Matching Molecular Orbital Energy Level Diagram For
Videos Matching Molecular Orbital Energy Level Diagram For
What Is The Bond Order Of He2 Quora
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
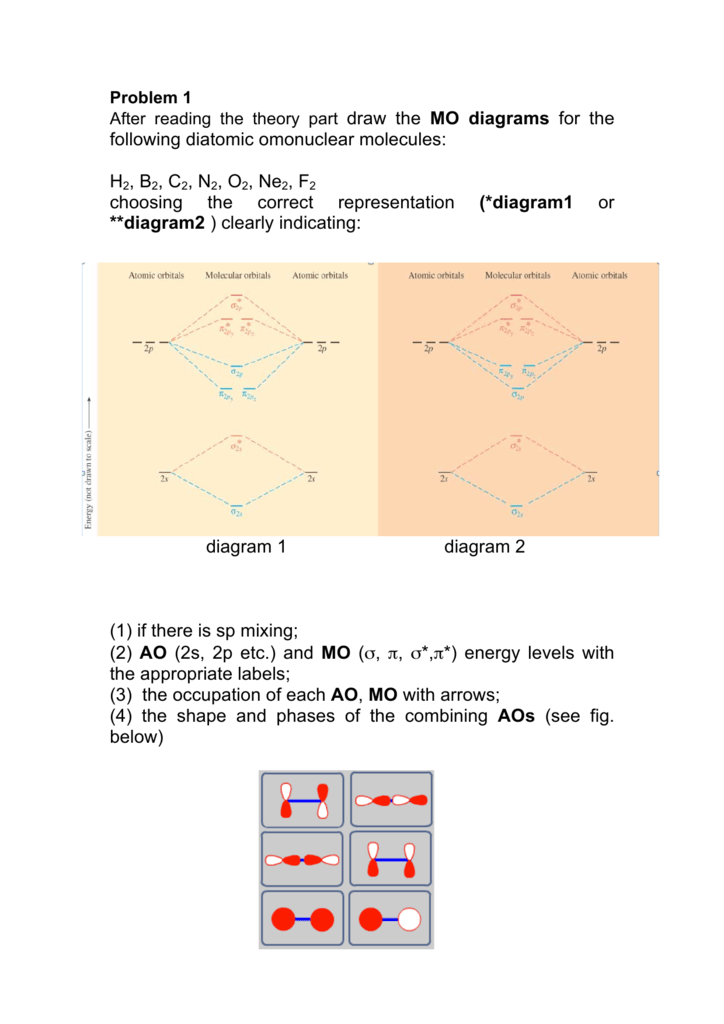 Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
 Chemical Bonding Molecular Orbitals Of H2 And He2
Chemical Bonding Molecular Orbitals Of H2 And He2
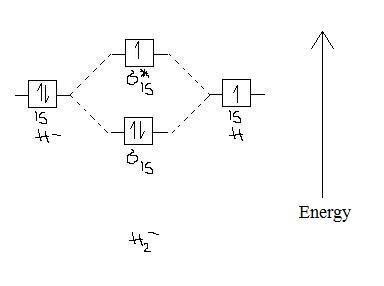 2 3b Mo Theory Of Bonding In H Chemistry Libretexts
2 3b Mo Theory Of Bonding In H Chemistry Libretexts
 Get Answer Construct The Molecular Orbital Diagram For
Get Answer Construct The Molecular Orbital Diagram For
 Energy Level Diagram For Molecular Orbitals Chemical
Energy Level Diagram For Molecular Orbitals Chemical
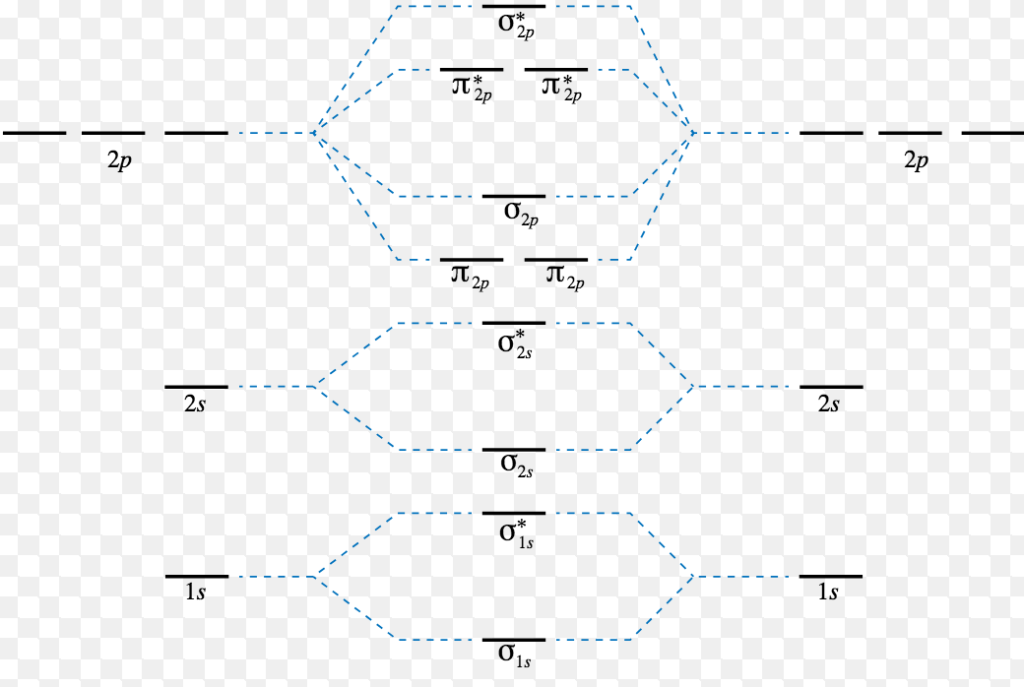 Energy Level Diagram For Molecular Orbitals Chemical
Energy Level Diagram For Molecular Orbitals Chemical
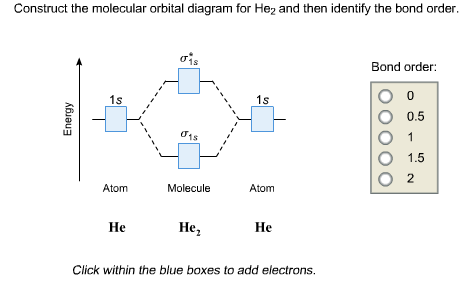 Solved Construct The Molecular Orbital Diagram For He2 An
Solved Construct The Molecular Orbital Diagram For He2 An
Sparknotes Organic Chemistry Orbitals Problems Molecular
 Ppt Lecture 27 Molecular Orbital Theory Iii Powerpoint
Ppt Lecture 27 Molecular Orbital Theory Iii Powerpoint
 Mo Diagram For H2o Wiring Diagram Schematics
Mo Diagram For H2o Wiring Diagram Schematics
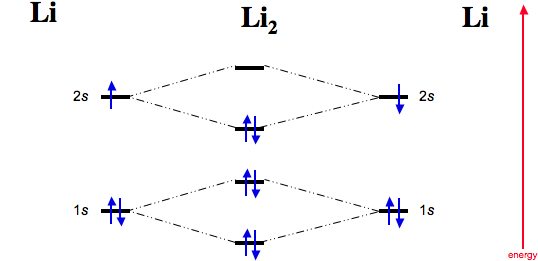 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
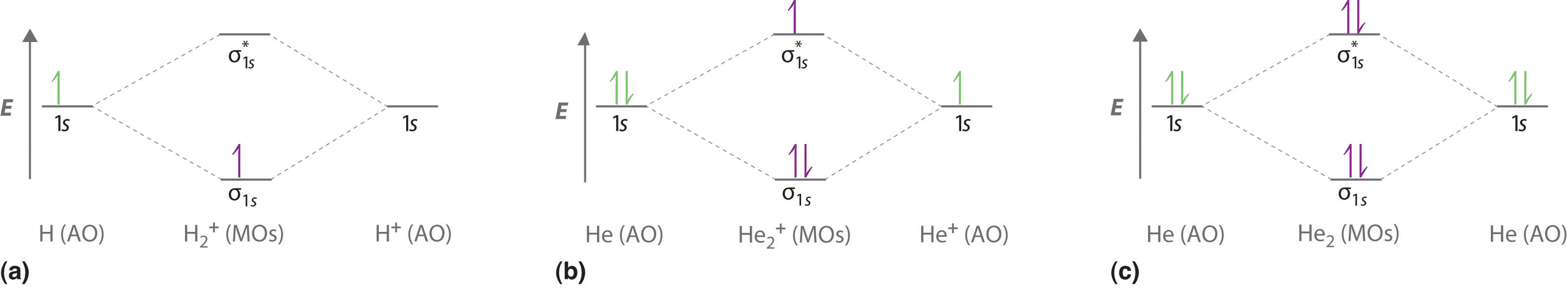 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
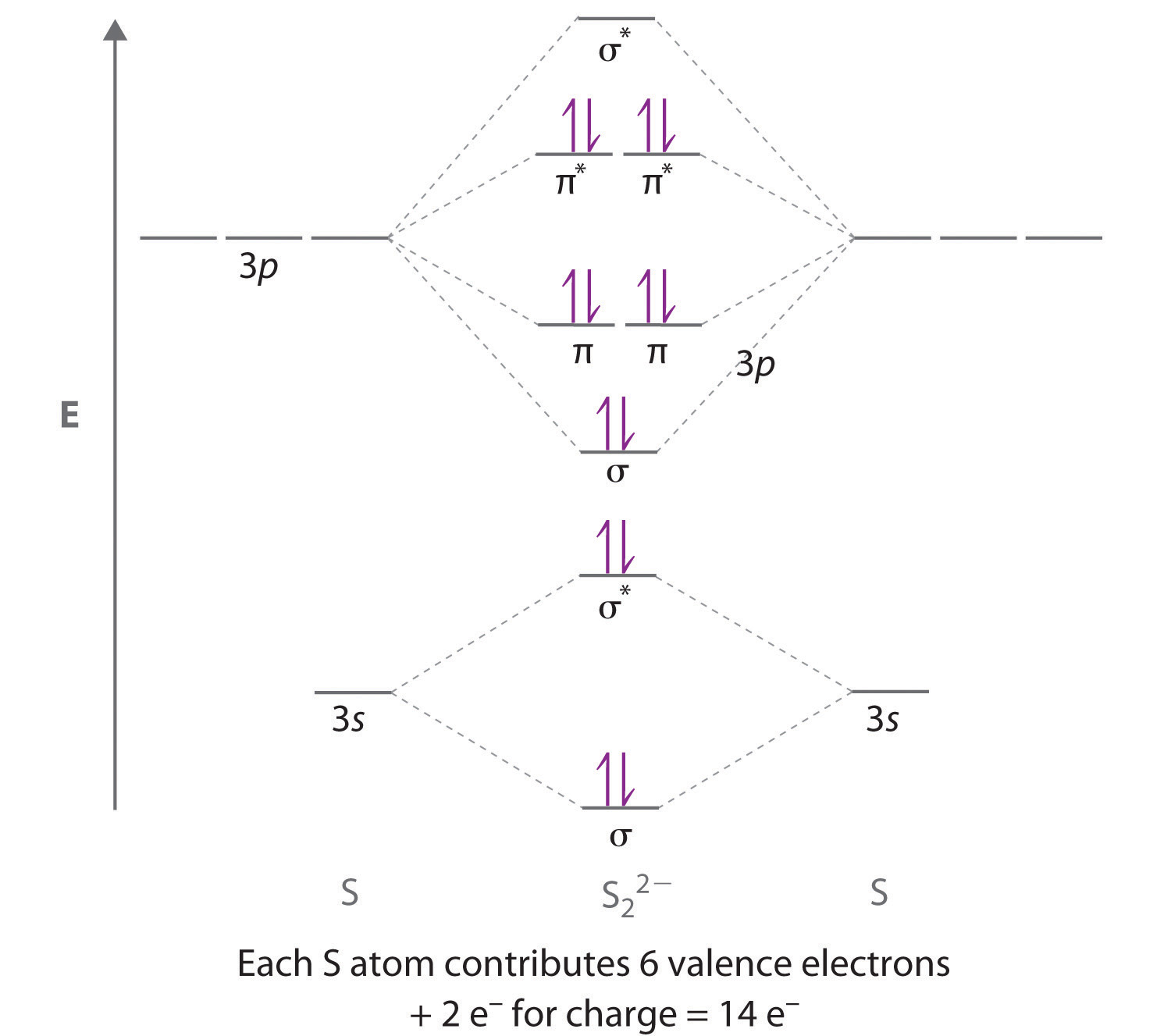 Chapter 6 5 Delocalized Bonding And Molecular Orbitals
Chapter 6 5 Delocalized Bonding And Molecular Orbitals
 Chemical Bonding Molecular Orbitals Of H2 And He2
Chemical Bonding Molecular Orbitals Of H2 And He2
 Energy Level Diagram For Molecular Orbitals Chemical
Energy Level Diagram For Molecular Orbitals Chemical
Schematic Molecular Orbital Diagram Of Planar Methane With

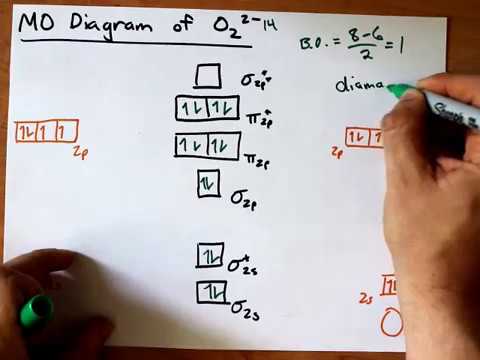
0 Response to "Molecular Orbital Diagram For He2 2"
Post a Comment