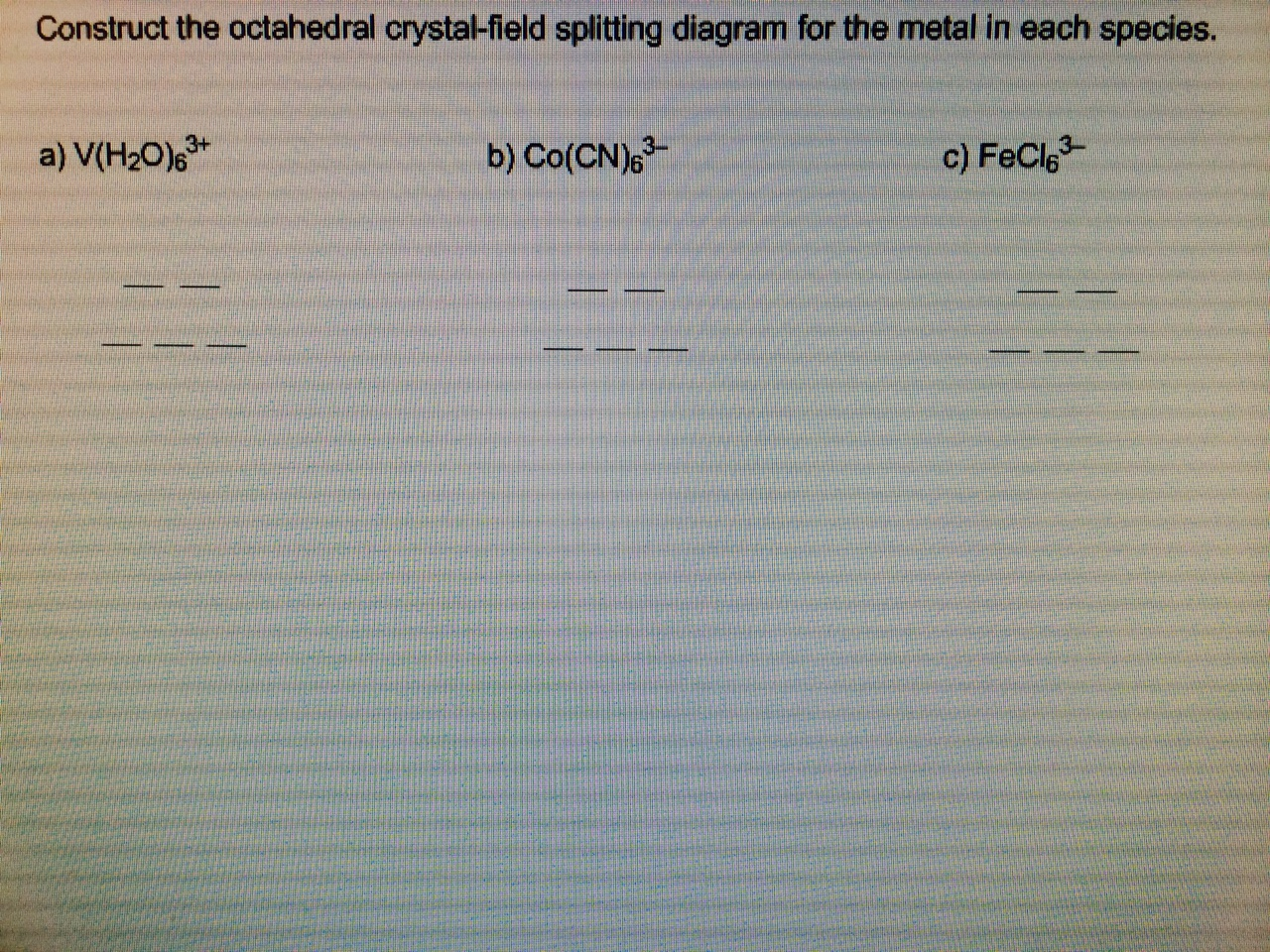
Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species
Based on crystal field theory which of the following metal ions will not be colored when placed in an octahedral crystal field. A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm.
 Rutgers Sas Grant Proposal For Instructional Computing
Rutgers Sas Grant Proposal For Instructional Computing
Construct the octahedral crystal field splitting diagram for the metal in each species.

Construct the octahedral crystal field splitting diagram for the metal in each species. A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm. Cr4 mnh2o62 20992 results page 20. How will the heat capacity of each metal sample afect.
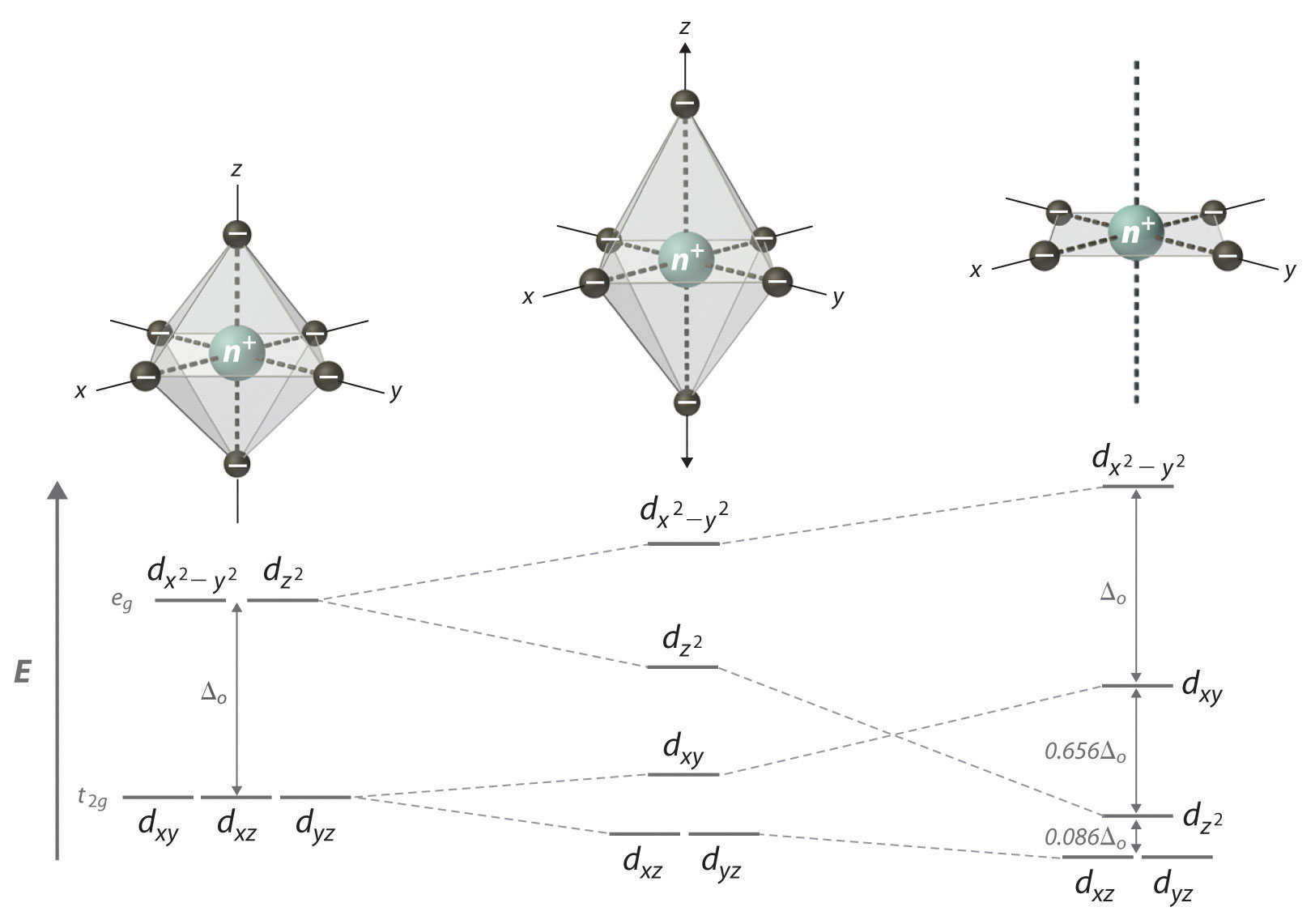
Just remember that color depends on which electronic transitions are available to the species in question. Crew b takes 8 hours to construct a wall of the same size. Answer to construct the octahedral crystal field splitting diagram for the metal in each species.
Vh2o63 cocn63 mnh2o62. It asks what is the electron configuration in this comound i got it to be d5. A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at nm.
Answer to construct the octahedral crystal field splitting diagram for the metal in each species. Construct the octahedral crystal field splitting diagram for the metal in each species. Basically the question is referring to the compound k3fec2o43.
You wrote in class we have only looked at the splitting of the orbitals not how things affect them you can explain all of these a c with crystal field theory. Fe in the compound is feiii so 23 electrons d5. It then asks how many unpaired electrons and asks to draw a crystal field splitting diagram for this compound.
A vh2083 b cocn8 c mnh2o. Construct the octahedral crystal field splitting diagram for the metal in each species. Vh2o63 cocn63 mnh2o62.
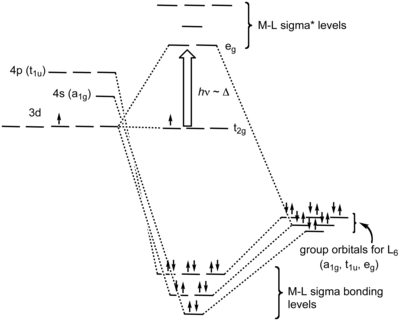
Hi all im stuck on this question and was wondering if anyone can help me figure it out. 3 for each species molecule or ion in the net ionic equation assign oxidation. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital.
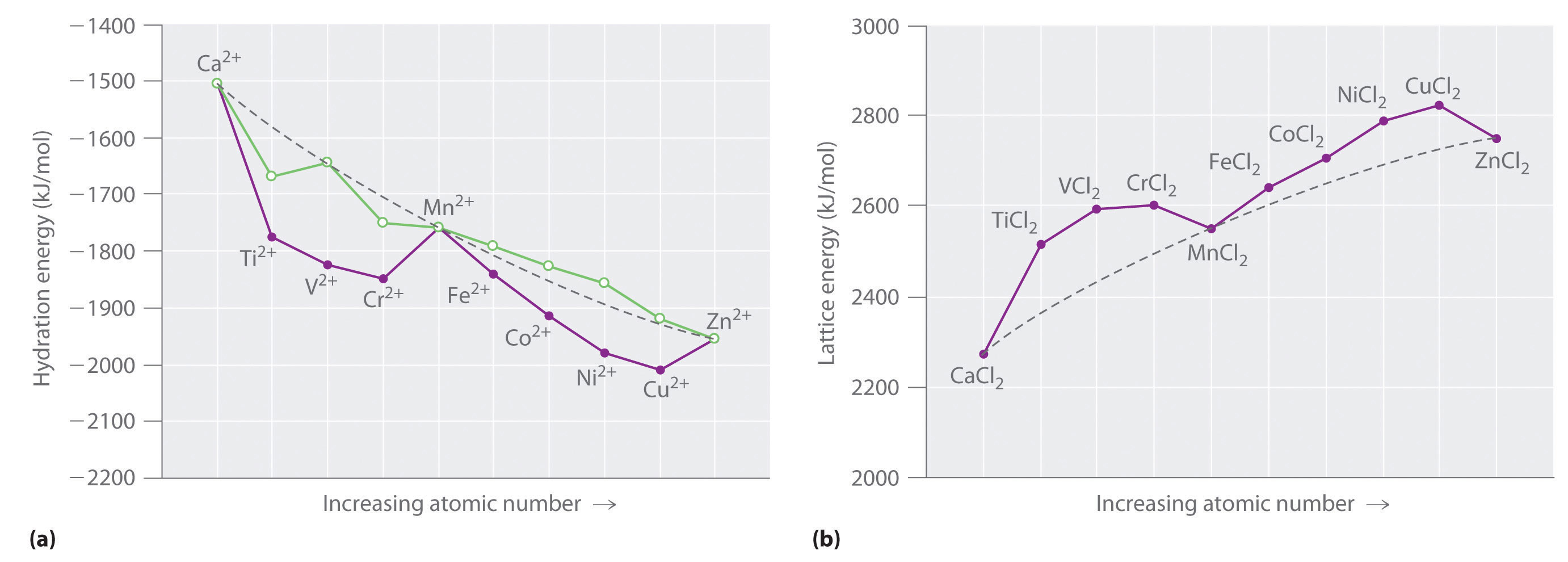
Au fe3 ag au3 nb3 asked by melody on november 9 2011. Solve a contractor finds that it takes a crew a 6 hours to construct a wall of a certain size. Conversely the dconversely the dx2 y22 and the dxy orbitals increase in energy the splitting orbitals increase in energy.
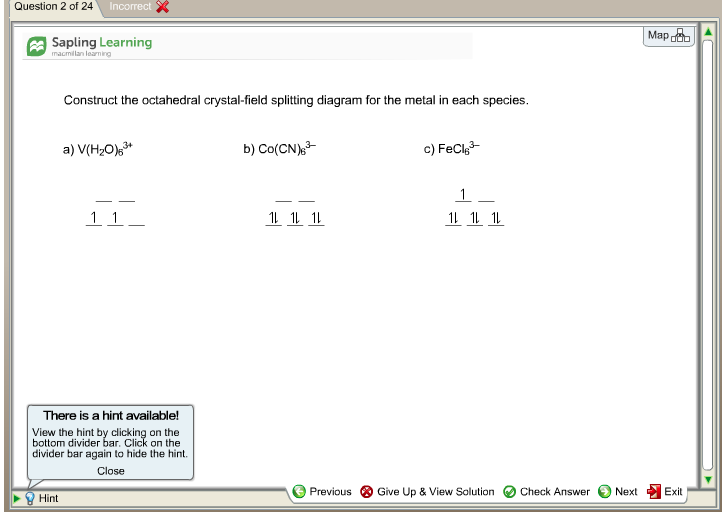 Construct The Octahedral Crystal Field Splitting Diagram For
Construct The Octahedral Crystal Field Splitting Diagram For
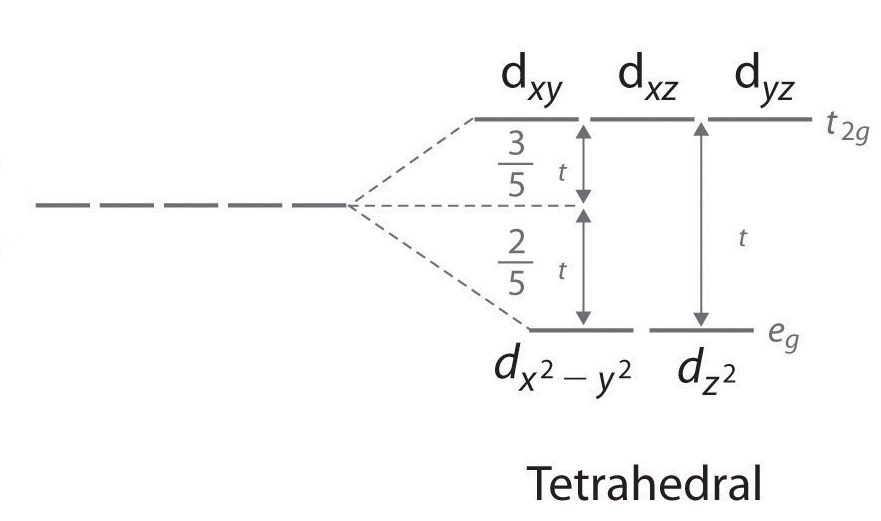 High Spin And Low Spin Complexes Chemistry Libretexts
High Spin And Low Spin Complexes Chemistry Libretexts
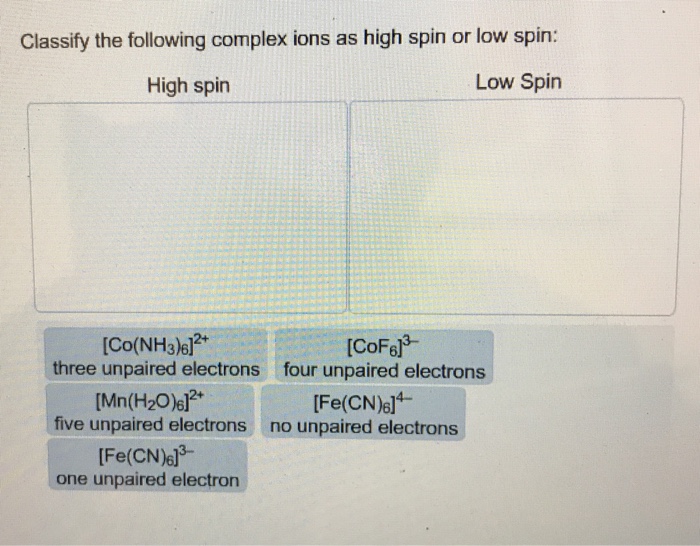
Chapter 20 D Block Metal Chemistry Coordination Complexes
University Of The West Indies Mona Jamaica
Course 201n 1 Semester 2006 2007 Inorganic Chemistry
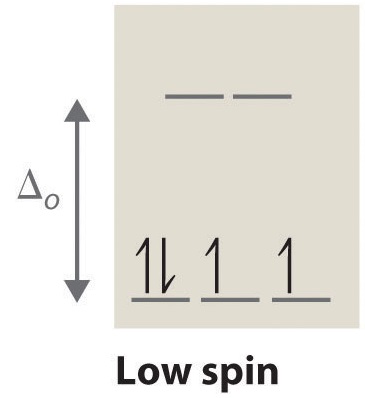 High Spin And Low Spin Complexes Chemistry Libretexts
High Spin And Low Spin Complexes Chemistry Libretexts
Topic 6 Coordination Compounds Coordination Chemistry
Topic 6 Coordination Compounds Coordination Chemistry

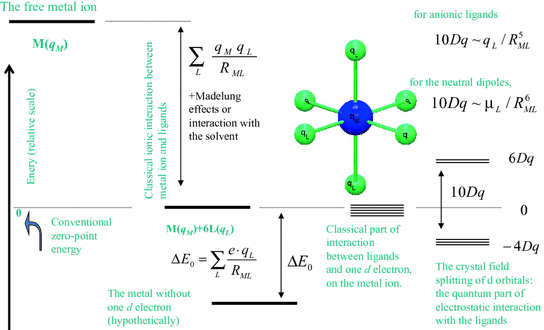
 A Modern First Principles View On Ligand Field Theory
A Modern First Principles View On Ligand Field Theory
Derivation Of The Aom Parameterization From The Effective
 Construct The Octahedral Crystal Field Splitting D
Construct The Octahedral Crystal Field Splitting D
 Introduction To Inorganic Chemistry Coordination Chemistry
Introduction To Inorganic Chemistry Coordination Chemistry
 Coordination Bonding Electronic Structure And Properties
Coordination Bonding Electronic Structure And Properties
 Ligand Field Splitting In Homoleptic Tetrahedral D10
Ligand Field Splitting In Homoleptic Tetrahedral D10
 Inorganic Chemistry What Does The Crystal Field Splitting
Inorganic Chemistry What Does The Crystal Field Splitting
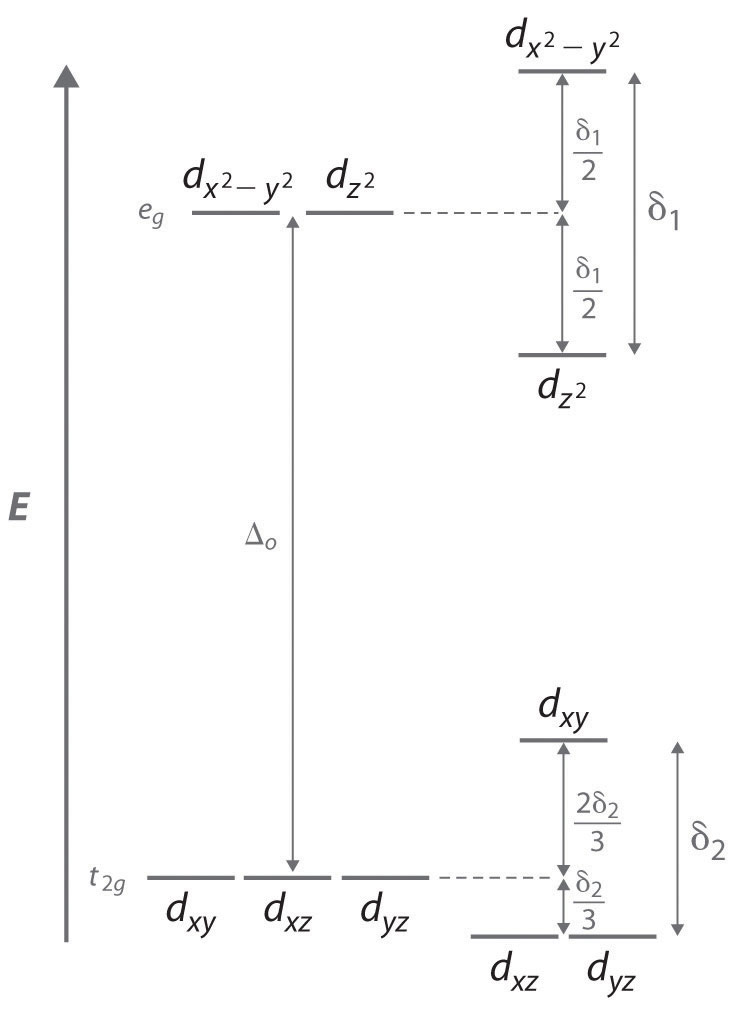
 Jahn Teller Effect In High Spin D4 And D9 Octahedral Metal
Jahn Teller Effect In High Spin D4 And D9 Octahedral Metal
0 Response to "Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species"
Post a Comment