Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion
Energy level diagram for thehe2 ion. If the bond order is greater than 0 we expect a bond to exist and the ion is stable.
University Of Groningen Generation And Interactions Of
One more electron in bonding than antibonding.
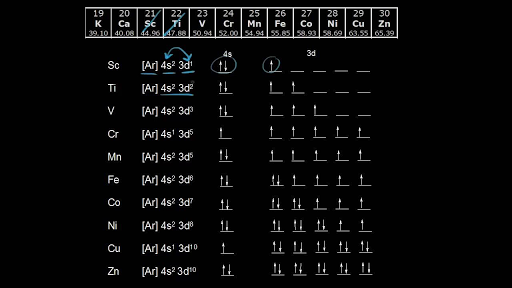
Which electrons in this diagram contribute to the stability of the he2 ion. Solved which electrons in this diagram contribute to the answer to which electrons in this diagram contribute to the stability of the ion a e electron in the sigma 1s mo and another o which of the following are predicted by the molecular o2 2 f2 2 h2 he2 home which of the following are predicted by the molecular electrons as needed here s the mo diagram. Please note the diagram is for he2 but the he h is very similar eg. If you add in two electrons they both go into the sigma anti bonding orbital and as a result the net bond order is zero.
He h forms a very weak bond. This mix to form a sigma orbital from h1sli2s a sigma orbital and h1s li2s. Li has 1s 2s while h has 1s.
Which electrons in this diagram contribute to the stability of the ion. The multiplicity of the bond is still determined by the fundamental rule of the octet. Which electrons in this diagram contribute to the stability of the he2 ion entitled as molecules free full text which electrons in this diagram contribute to the stability of the he2 ion.
Also describes molecules free full text and labeled as. Energy level diagram for the he 2 ion. The assumption that the bond in he2 is three electron is incorrect.
The two electrons in the sigma 1s mo. One electron in the sigma 1s mo and another one in the sigma 1s mo. Therefore in he2 there are 3 electrons.
With resolution 3400px x 2481px. Therefore it is easy to show that the bond in he2 is one electron hence its multiplicity is 05. No it only has one atom but a polyatomic ions have more than one.
One electron in the sigma 1s mo and another one in the sigma 1s mo. F22 is not stable. When an atom loses electrons the ion that is formed has a positive charge.
The electron in the sigma 1s mo. The two electrons in the sigma 1s mo. The valence electrons of he are in the 1s orbital and the 1s orbitals combine to give an mo diagram like that for h 2 or he 2 figure 933.
Do monatomic ions consist of more than one atom. He2 would have a bond order of zero but he2 would have a bond order of 05 2 e in the bonding orbital and 1 electron in the anti bonding orbital. 1 answer to which electrons in this diagram contribute to the stability of the ion.
Energy level diagram for the he2 ionwhich electrons in this diagram contribute to the stability of the he2 ion. Energy level diagram for the he2 ionwhich electrons in this diagram contribute to the stability of the he2 ion.
 Ignition And Afterglow Dynamics Of A High Pressure
Ignition And Afterglow Dynamics Of A High Pressure
 Potential Energy Diagrams For Formation Of Bonds Mini
Potential Energy Diagrams For Formation Of Bonds Mini
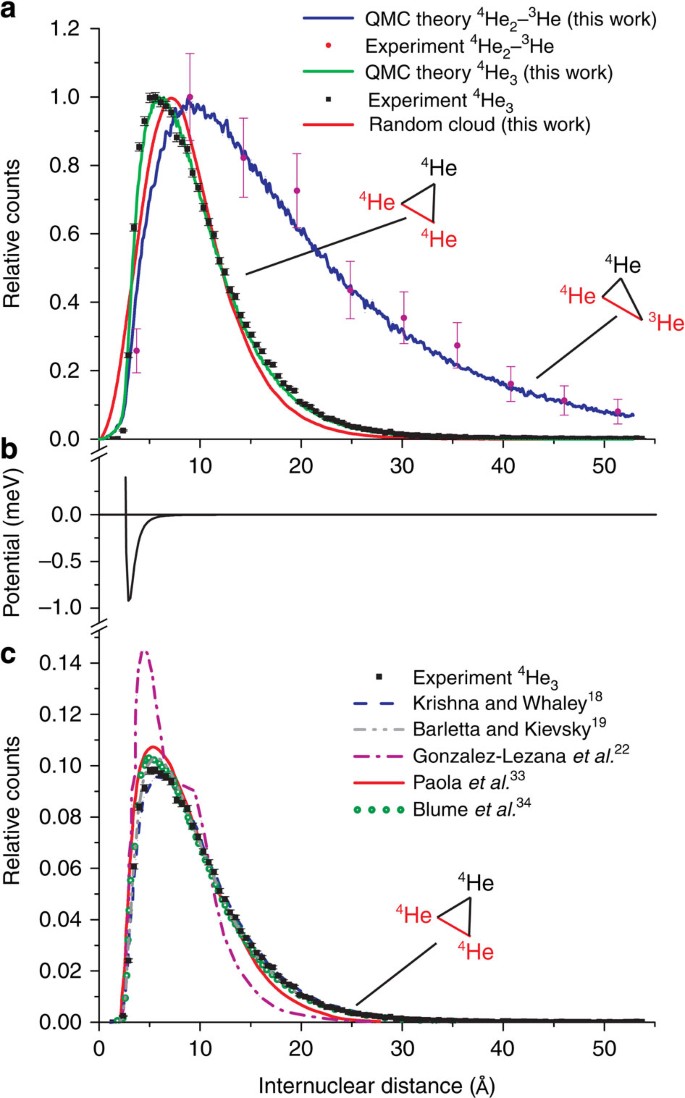 Imaging The Structure Of The Trimer Systems 4 He 3 And 3 He
Imaging The Structure Of The Trimer Systems 4 He 3 And 3 He
Which Is More Stable He2 Or H2 And Why Quora
 Helium Definition Properties Uses Facts Britannica Com
Helium Definition Properties Uses Facts Britannica Com
 Alterations In The Human Blood Epididymis Barrier In
Alterations In The Human Blood Epididymis Barrier In
Spatio Temporal Averaged Power Densities Coupled Into
 Molecular Orbital Theory Bonding Antibonding Mo Bond Order Homonuclear Diatomic Molecules
Molecular Orbital Theory Bonding Antibonding Mo Bond Order Homonuclear Diatomic Molecules
 Potential Energy Diagrams For Formation Of Bonds Mini
Potential Energy Diagrams For Formation Of Bonds Mini
 Atomic Structure Electrons Protons Neutrons And Atomic
Atomic Structure Electrons Protons Neutrons And Atomic
 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
Orbital Interactions In Chemistry
Which Is More Stable He2 Or H2 And Why Quora
 Pdf Magnetosheath Compression Role Of Characteristic
Pdf Magnetosheath Compression Role Of Characteristic

 Molecular Orbital Mo Diagram Of Be2
Molecular Orbital Mo Diagram Of Be2
 Energy Level Diagram For The He2 Ion Whic Clutch Prep
Energy Level Diagram For The He2 Ion Whic Clutch Prep
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 9 3e Drawing The Mo Energy Diagram For A Period 2 Homodiatom
9 3e Drawing The Mo Energy Diagram For A Period 2 Homodiatom
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Stabilization Of The C20 Cage By Encapsulation Of H And
0 Response to "Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion"
Post a Comment