Electron Dot Diagram For Fluorine
What is the correct lewis structure for fluorine which is a group 7a element. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital.
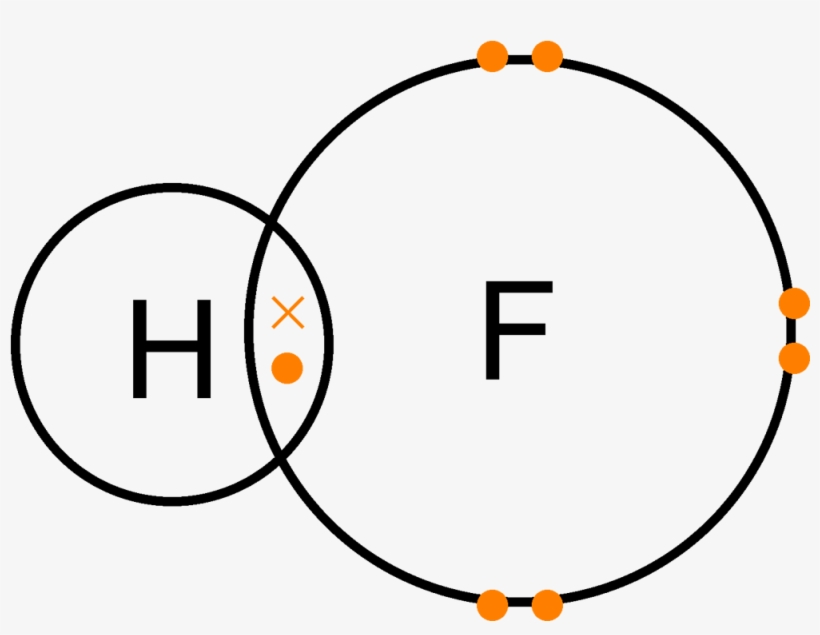 Dot Diagram Fluorine Wiring Diagram
Dot Diagram Fluorine Wiring Diagram
And thus the neutral atom has 7 valence electrons.

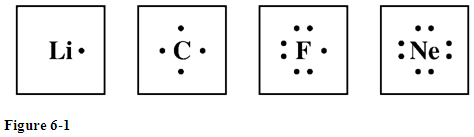
Electron dot diagram for fluorine. Lithium atom loses one electron to form the cation li 2. Once it reacts with a nonmetal to form fluoride fluorine with a negative 1 charge you can put the 8th electron on this diagram stability comes from filling up the val. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1.
Choose the statement that correctly identifies the most stable of the elements. It is f with two dots on the top two dots on the bottom two dots on one side and 1 dot on the other side. It is commonly shipped as a cryogenic liquid.
The lewis dot diagram for neon has a pair of electrons on each side of neon symbol ne for a total of 8 electrons. Since 1s can only hold two electrons the next 2 electrons for f go in the 2s orbital. Therefore the f electron configuration will be 1s 2 2s 2 2p 5.
Fluorine is a pale yellow gas with a pungent odor. It is toxic by inhalation and skin absorption. Which do you think would be bigger.
Lithium fluoride lif 1. The lewis structure electron dot diagram of each ion is used to construct the lewis structure electron dot diagram for the ionic compound. Upon reduction the fluorine atom forms fluoride which has 8 valence electrons and is isoelectronic with a noble gas which one.
I show you where fluorine is on the periodic table and how to determine how many valence electrons it has. Contact with skin in lower than lethal concentrations causes chemical burns. Choose the statement the correctly identifies the most stable of the elements.
There are 7 valence electrons in fluorine or any halogen for that matter. Thats a total of 7 dots electrons and that is the lewis structure for fluorine f. Fluorine is in group 17 of the periodic table.
The remaining five electrons will go in the 2p orbital. Of course the elemental form is bimolecular. Fluorine atom gains one electron to form the anion f 3.
A step by step explanation of how to draw the lewis dot structure for f fluorine. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1. Neon is the most stable element because its highest occupied energy level is filled.
Fluorine atom or fluoride ion. Lithium fluoride compound can be represented as li or 1.
 Help With Lewis Diagrams High School Chemistry
Help With Lewis Diagrams High School Chemistry
Dot Diagram Fluorine Catalogue Of Schemas
 How To Determine The Lewis Dot Structure For F2 Quora
How To Determine The Lewis Dot Structure For F2 Quora
Dot Diagram Fluorine N5 Electrical Schemes
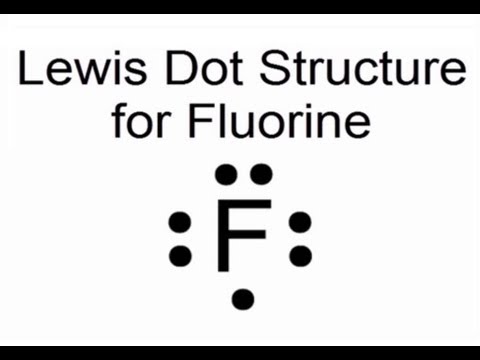 Lewis Dot Structure For Fluorine Atom F
Lewis Dot Structure For Fluorine Atom F
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
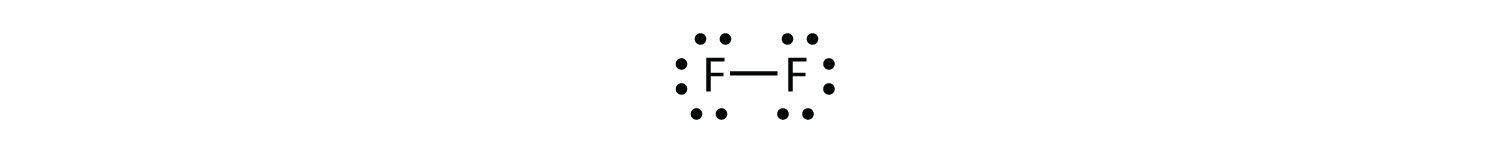 Dot Diagram Fluorine N5 Electrical Schemes
Dot Diagram Fluorine N5 Electrical Schemes
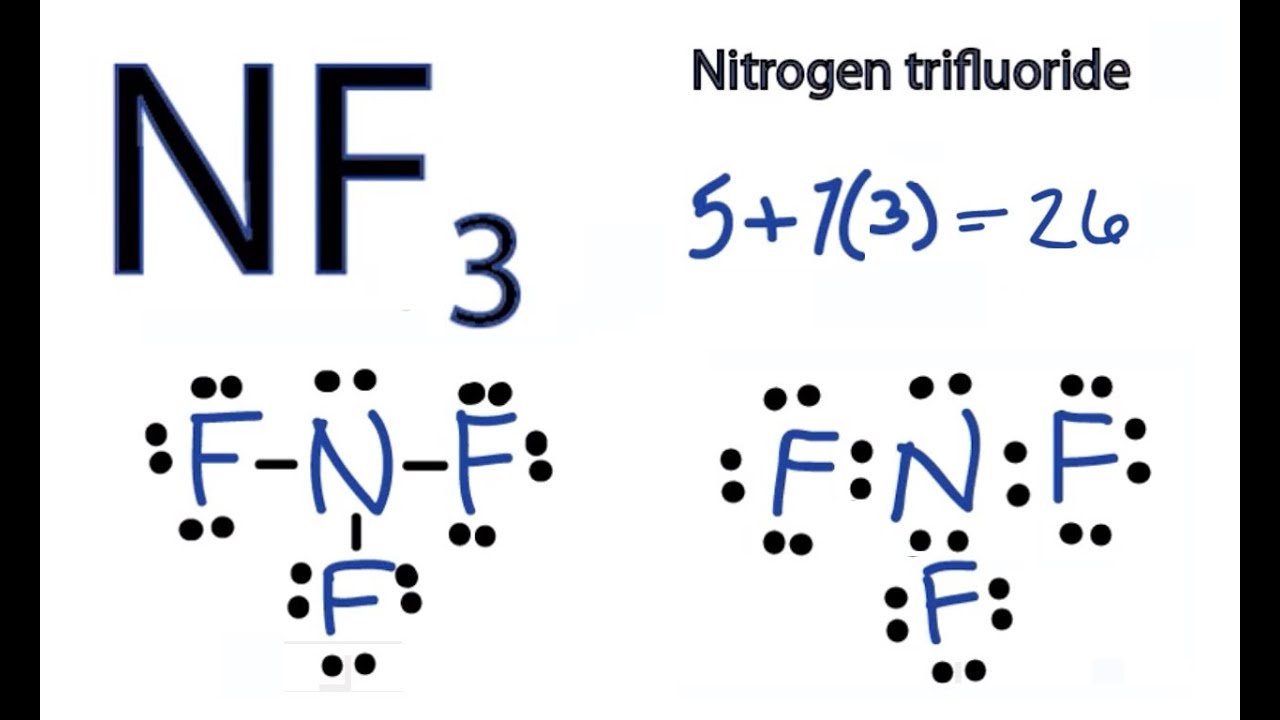 Nf3 Lewis Structure How To Draw The Dot Structure For Nf3 Nitrogen Trifluoride
Nf3 Lewis Structure How To Draw The Dot Structure For Nf3 Nitrogen Trifluoride
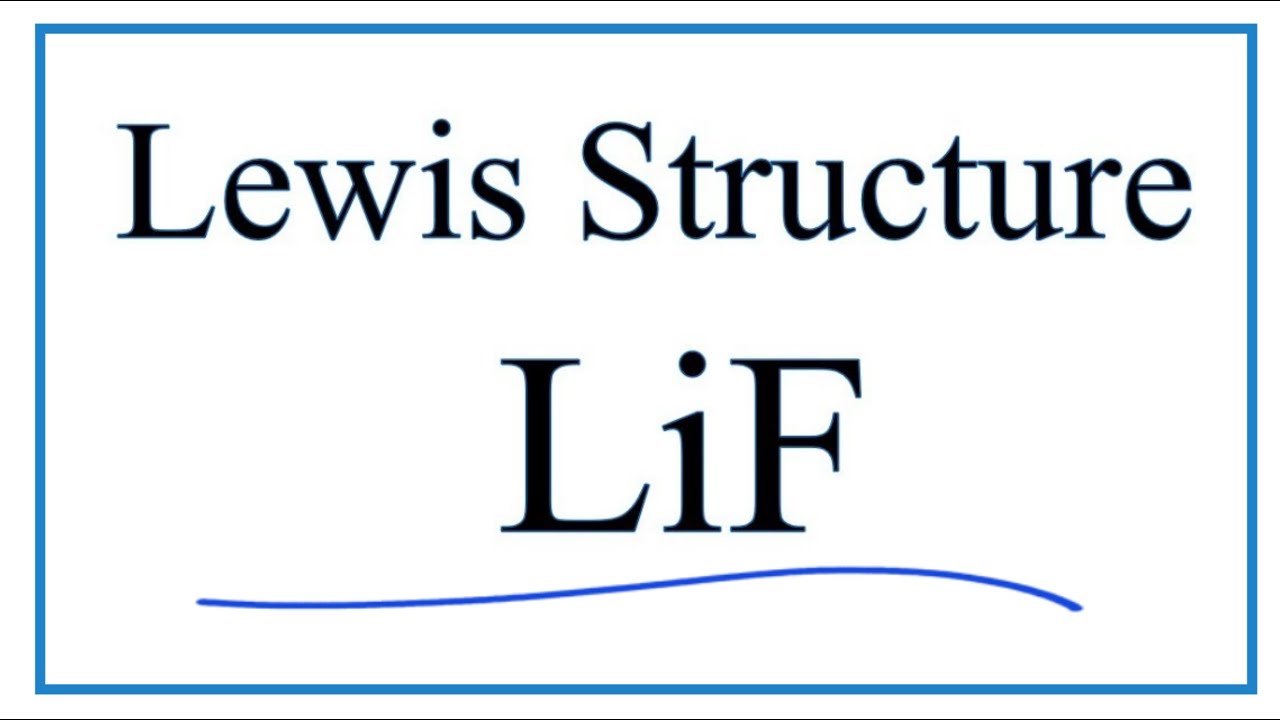 How To Draw The Lewis Dot Structure For Lif Lithium Fluoride
How To Draw The Lewis Dot Structure For Lif Lithium Fluoride
 How To Draw The Lewis Dot Structure For F Fluoride Ion
How To Draw The Lewis Dot Structure For F Fluoride Ion
 Lewis Structure And Bonding Lewis Dot Diagram Of Atoms The
Lewis Structure And Bonding Lewis Dot Diagram Of Atoms The
 What Type Of Hybridization Does The Central Atom In Sf 4
What Type Of Hybridization Does The Central Atom In Sf 4
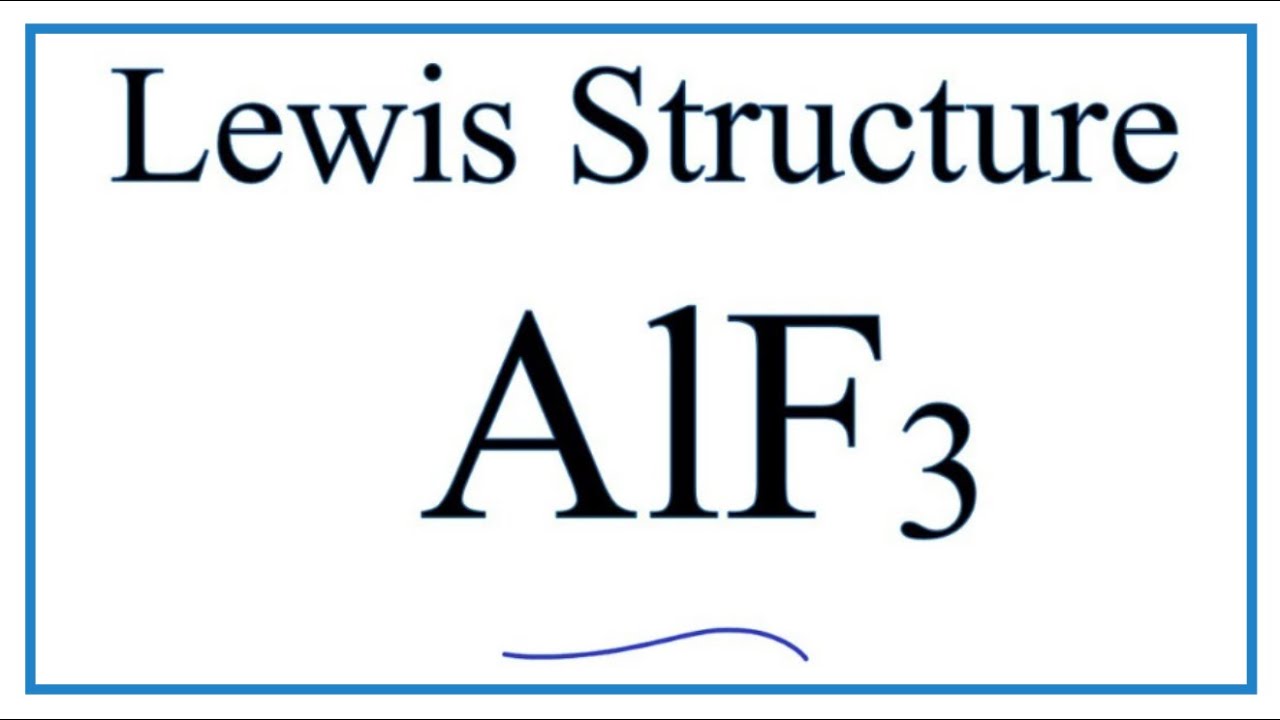 How To Draw The Lewis Dot Structure For Alf3 Aluminum Fluoride
How To Draw The Lewis Dot Structure For Alf3 Aluminum Fluoride
 Lewis Symbols And Structures Chem 1305 Introductory Chemistry
Lewis Symbols And Structures Chem 1305 Introductory Chemistry
 Why Is Boron Trifluoride Written In Two Ways In The Lewis
Why Is Boron Trifluoride Written In Two Ways In The Lewis
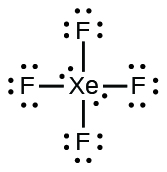 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry

 Lewis Structure For Of2 Oxygen Difluoride
Lewis Structure For Of2 Oxygen Difluoride
 Chapter 5 2 Lewis Electron Dot Symbols Chemistry Libretexts
Chapter 5 2 Lewis Electron Dot Symbols Chemistry Libretexts
 Lewis Symbols And Structures Chem 1305 Introductory Chemistry
Lewis Symbols And Structures Chem 1305 Introductory Chemistry
 Solved Study The Electron Dot Diagrams For Lithium Carbo
Solved Study The Electron Dot Diagrams For Lithium Carbo
 Dot Diagram Fluorine N5 Electrical Schemes
Dot Diagram Fluorine N5 Electrical Schemes



0 Response to "Electron Dot Diagram For Fluorine"
Post a Comment