Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion
Co2 high spin this problem has been solved. Splitting of the d orbitals in an octahedral field consequences of d orbital splitting.
Mn high and low spin d.
Draw the octahedral crystal field splitting diagram for each metal ion. Which of the following are true for the crystal field model ofan octahedral complex ion. Draw the octahedral crystal field splitting diagram for each metal ion. Consider ions to be from first rowtransition metals.
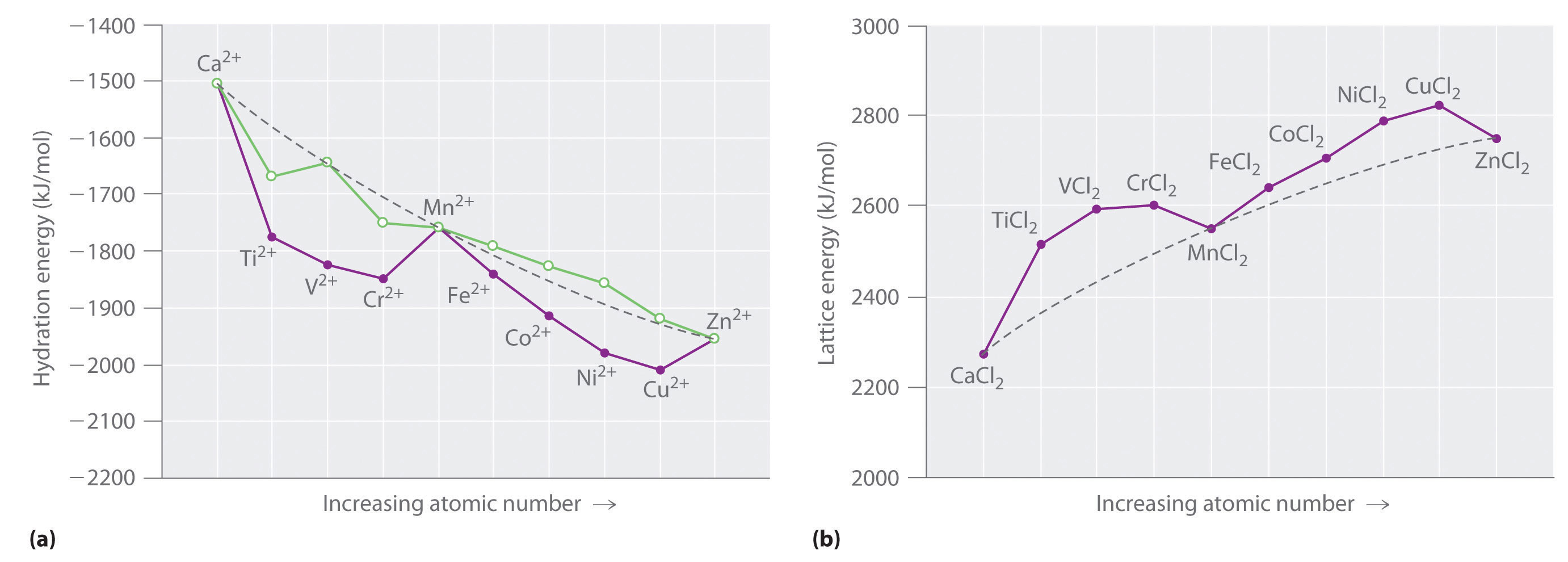
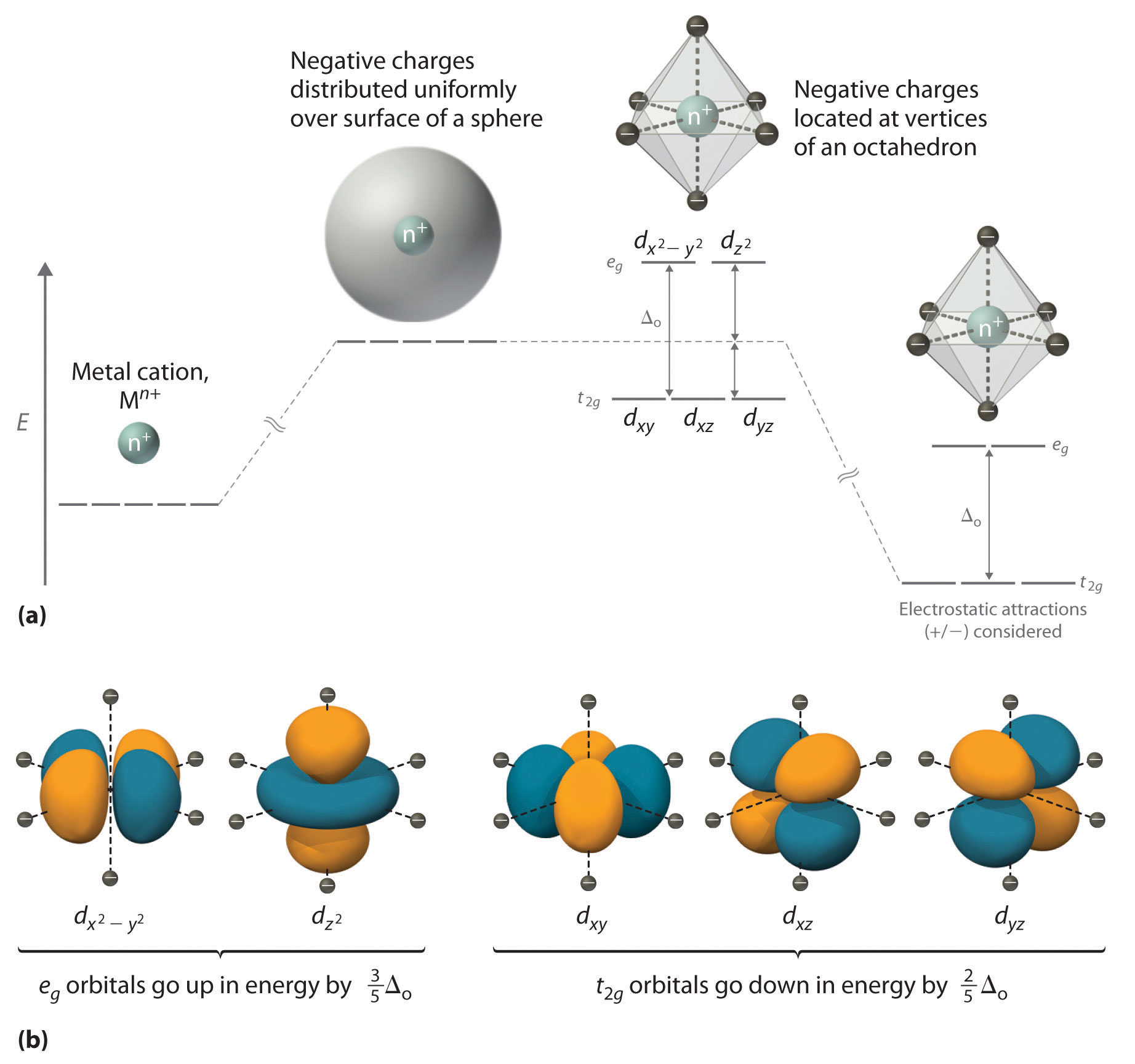
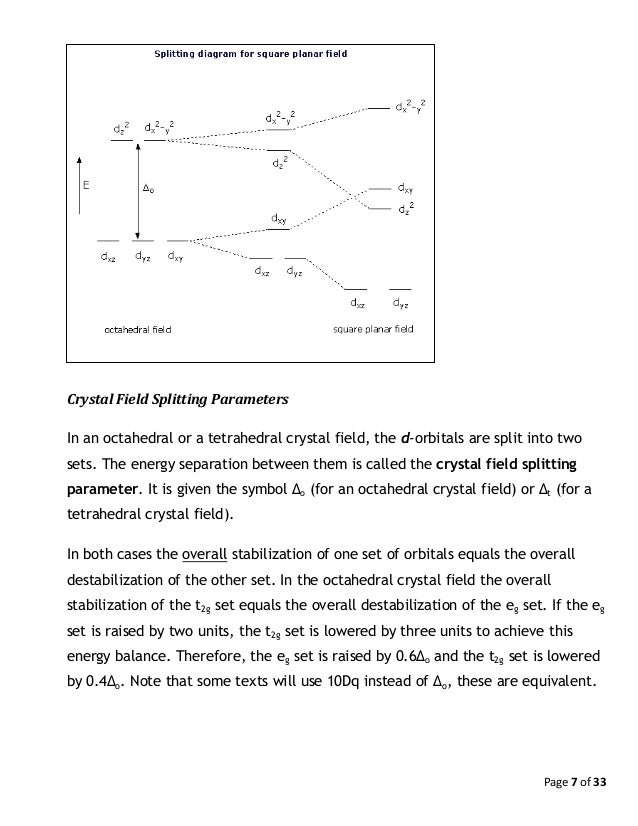
Color lecture 7 crystal field theory for octahedral complexes boats and propellersif you have a single engine inboard installation the stern will pull to port left when you go into reverse if you have a. 1 draw the octahedral crystal field splitting diagram for each metal ion. The d x 2 y 2 and d z 2 orbitals on the metal ion at the center of the cube lie between the ligands and the d xy d xz and d yz orbitals point toward the ligands.
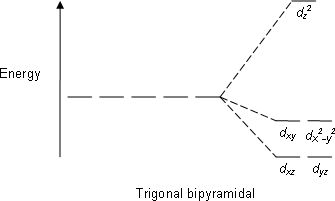
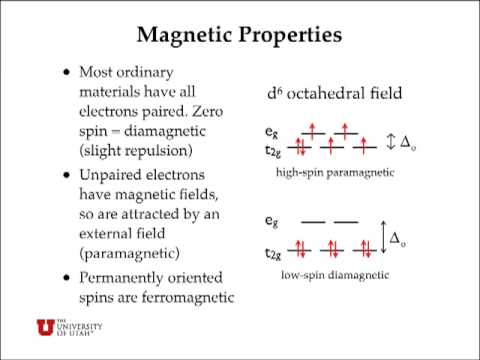
A how many unpaired electrons are in the d orbital splitting diagram for the octahedral complex ion of co2 low spin b how many unpaired electrons are in the following complex ion runh362 lowspin. Cr3 cu2 mn3 high spin mn3 low spin fe2 low spin this problem has been solved. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex.
It then asks how many unpaired electrons and asks to draw a crystal field splitting diagram for this compound. This chemistry video tutorial provides a basic introduction into crystal field theory. Draw the octahedral crystal field splitting diagram for each metal ion.
It asks what is the electron configuration in this comound i got it to be d5. Fe3 high and low spin d. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy δ o whose magnitude depends on the charge on the metal ion the position of the metal in the periodic table and the nature of the ligands.
Get the detailed answer. The spin pairing energy p is the increase in energy that occurs when an electron is added. Hi all im stuck on this question and was wondering if anyone can help me figure it out.
Basically the question is referring to the compound k3fec2o43. Magnetism consequences of d orbital splitting. It explains how to draw the crystal field splitting diagram of transition metal complex ions using weak field.
Construct the octahedral crystal field splitting diagram for the metal in. Fe in the compound is feiii so 23 electrons d5. 1 answer to draw and label the crystal field splitting diagram for the 3d atomic orbitals in an octahedral field 113027.

Coordination Chemistry Ii Ligand Field Theory Continued
 Crystal Field Splitting For The Octahedral Coordinated Co 3
Crystal Field Splitting For The Octahedral Coordinated Co 3
 Introduction To Crystal Field Theory Chemistry Libretexts
Introduction To Crystal Field Theory Chemistry Libretexts
 Ligand Field Theory An Overview Sciencedirect Topics
Ligand Field Theory An Overview Sciencedirect Topics
Chemistry The Central Science Chapter 24 Section 5
Chemistry The Central Science Chapter 24 Section 5
 Crystal Field Theory Wikipedia
Crystal Field Theory Wikipedia
 Crystal Field Theory Wikipedia
Crystal Field Theory Wikipedia
Chemistry The Central Science Chapter 24 Section 5

 Coordination Compounds In An Octahedral Complex What
Coordination Compounds In An Octahedral Complex What
Topic 6 Coordination Compounds Coordination Chemistry
 Introduction To Inorganic Chemistry Coordination Chemistry
Introduction To Inorganic Chemistry Coordination Chemistry
 Which Of These Ions Will Have A Zero Crystal Field Splitting
Which Of These Ions Will Have A Zero Crystal Field Splitting
 Crystal Field Theory Octahedral Geometry For Coordination Compounds
Crystal Field Theory Octahedral Geometry For Coordination Compounds
 Crystal Field Theory Chemistry Libretexts
Crystal Field Theory Chemistry Libretexts

 Crystal Field Theory Wikipedia
Crystal Field Theory Wikipedia
 Inorganic Chemistry Why Do Octahedral Metal Ligand
Inorganic Chemistry Why Do Octahedral Metal Ligand
 4 Splitting Of D Orbitals In An Octahedral Ligand Field A
4 Splitting Of D Orbitals In An Octahedral Ligand Field A
Interpretation Of The Spectra Of First Row Transition Metal
 Bonding In Coordination Compounds Crystal Field Theory
Bonding In Coordination Compounds Crystal Field Theory
 Why Spliting Of D Orbital In Tetrahedral Crystal Field
Why Spliting Of D Orbital In Tetrahedral Crystal Field
0 Response to "Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion"
Post a Comment