What Is The Basis For Exceptions To The Aufbau Diagram
For example ruthenium rhodium silver and platinum are all exceptions to the aufbau principle because of filled or half filled subshells. Some elements have unusual atomic orbitals.
 Electron Configuration Exceptions Copper Cu And Chromium Cr
Electron Configuration Exceptions Copper Cu And Chromium Cr
But because half filled orbitals and complete orbitals are more stable an.
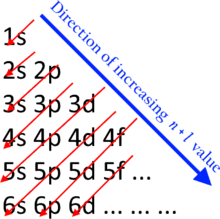
What is the basis for exceptions to the aufbau diagram. He theorized that electrons traveled in. D4 d9 f6 or f13. What is the basis for exceptions to the aufbau principle.
Start studying chemistry chapter 5. Filled and half filled energy sublevels are more stable than partially filled energy sublevels. Filled and half filled energy sublevels are more stable than partially filled energy sublevels.
Despite the exceptions the aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements. But a more complicated way of how the aufbau principle is disregarded is when there is hybridization of the orbitals together. According to the aufbau principle.
Exceptions to the aufbau principle by james ruybal. What is the basis for exceptions to the aufbau diagram. Other exceptions are copper and silver.
What is the basis for exceptions to the aufbau diagrams. You get s orbitals and p orbitals fuzing to create sp sp2 and sp3 orbitals. A chart or diagram may be used to show how the principle works for various example elements.
But dealing with those are only needed when knowing the actual 3 d shape of the molecule. What is the basis for exceptions to the aufbau diagram. Who discovered the aufbau principle.
Filled and half filled energy sublevels are more stable than partially filled energy sublevels. What is the basis for exceptions to the aufbau diagram. For example for cu with z29 if we follow aufbau its condensed electronic config should be ar 4s ² 3d⁹.
This exception is only applied when the configuration sequence ends with. The simple answer to your question is just putting unidirectional electrons in the p orbital. The examples above copper and silver end with d4 and d9 making them eligible for the exception.
Some transition metals dont follow the aufbau because half filled orbitals and completely filled orbitals are associated with the stability of the element. In the lower atomic numbers the difference in energy levels for the normal sequence of electron shells is larger and exceptions are not as common. Which scientist developed the quantum mechanical model of the atom.
Bohr stoner suggested aufbau principle. A filled and half filled energy sublevels are more stable than partially filled energy sublevels b electron configurations are only probable. Learn vocabulary terms and more with flashcards games and other study tools.
 The Trouble With The Aufbau Principle Feature Education
The Trouble With The Aufbau Principle Feature Education
 Pointillist Rendering Of Electron Charge And Spin Density
Pointillist Rendering Of Electron Charge And Spin Density
 Building Up The Periodic Table
Building Up The Periodic Table
Electronic Structure Of Atoms Electron Configurations

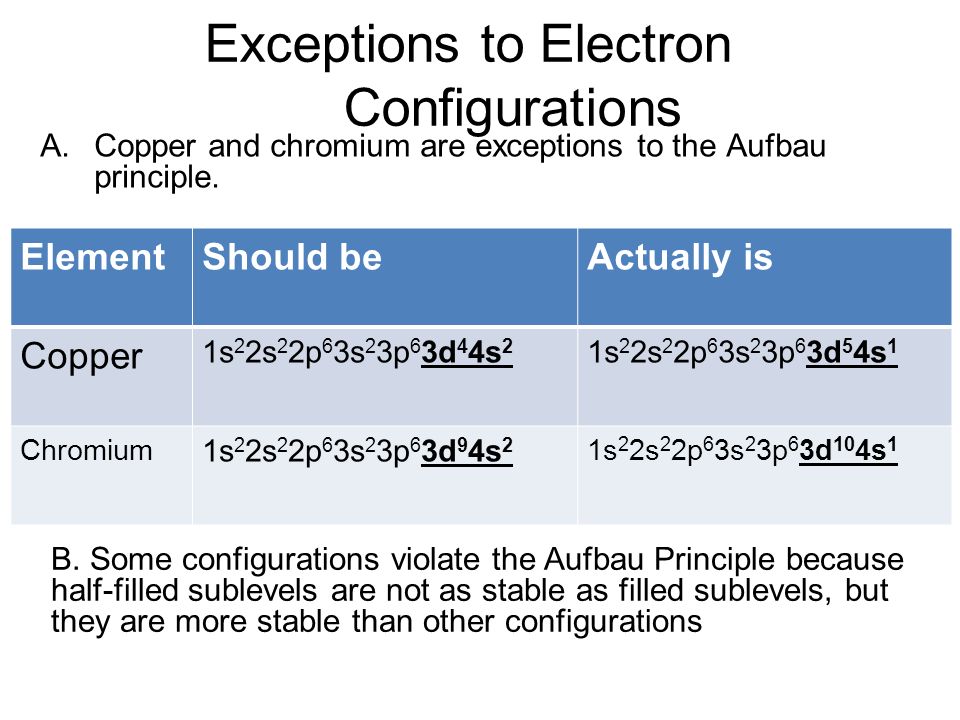 Aufbau Diagram Copper Wiring Diagram
Aufbau Diagram Copper Wiring Diagram
 What Is The Basis For Exceptions To The Aufbau Principle
What Is The Basis For Exceptions To The Aufbau Principle
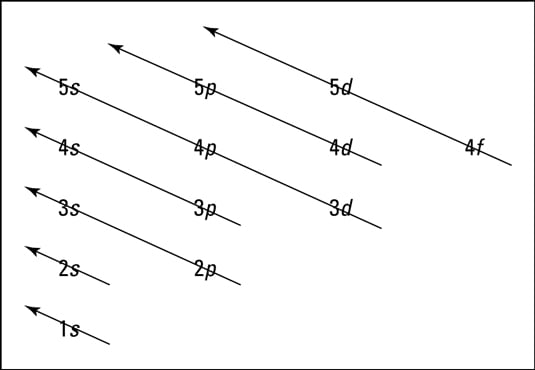 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
 What Is The Basis For Exceptions To The Aufbau Principle
What Is The Basis For Exceptions To The Aufbau Principle
 Ppt Chapter 5 Electrons In Atoms Powerpoint Presentation
Ppt Chapter 5 Electrons In Atoms Powerpoint Presentation
 6 4 Electronic Structure Of Atoms Electron Configurations
6 4 Electronic Structure Of Atoms Electron Configurations
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 The Pauli Exclusion Principle Physics
The Pauli Exclusion Principle Physics
 Half Filled And Full Filled Subshell Writing Electronic Configuration Ashwin Sir Ashwin Sir
Half Filled And Full Filled Subshell Writing Electronic Configuration Ashwin Sir Ashwin Sir
Causal Explanation And The Periodic Table
 How Do Chromium And Copper Contradict The Aufbau Principle
How Do Chromium And Copper Contradict The Aufbau Principle
 A Linear Cobalt Ii Complex With Maximal Orbital Angular
A Linear Cobalt Ii Complex With Maximal Orbital Angular
 Pdf The Full Story Of The Electron Configurations Of The
Pdf The Full Story Of The Electron Configurations Of The
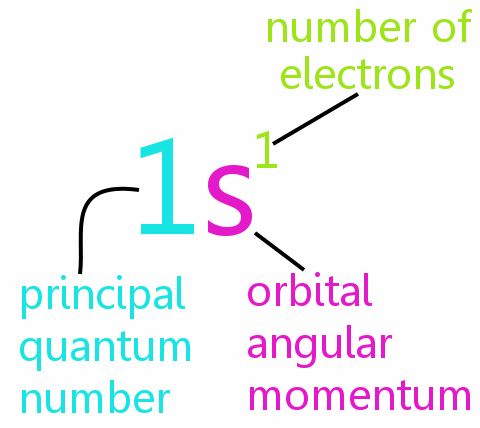 Definition Of The Pauli Exclusion Principle Chemistry
Definition Of The Pauli Exclusion Principle Chemistry
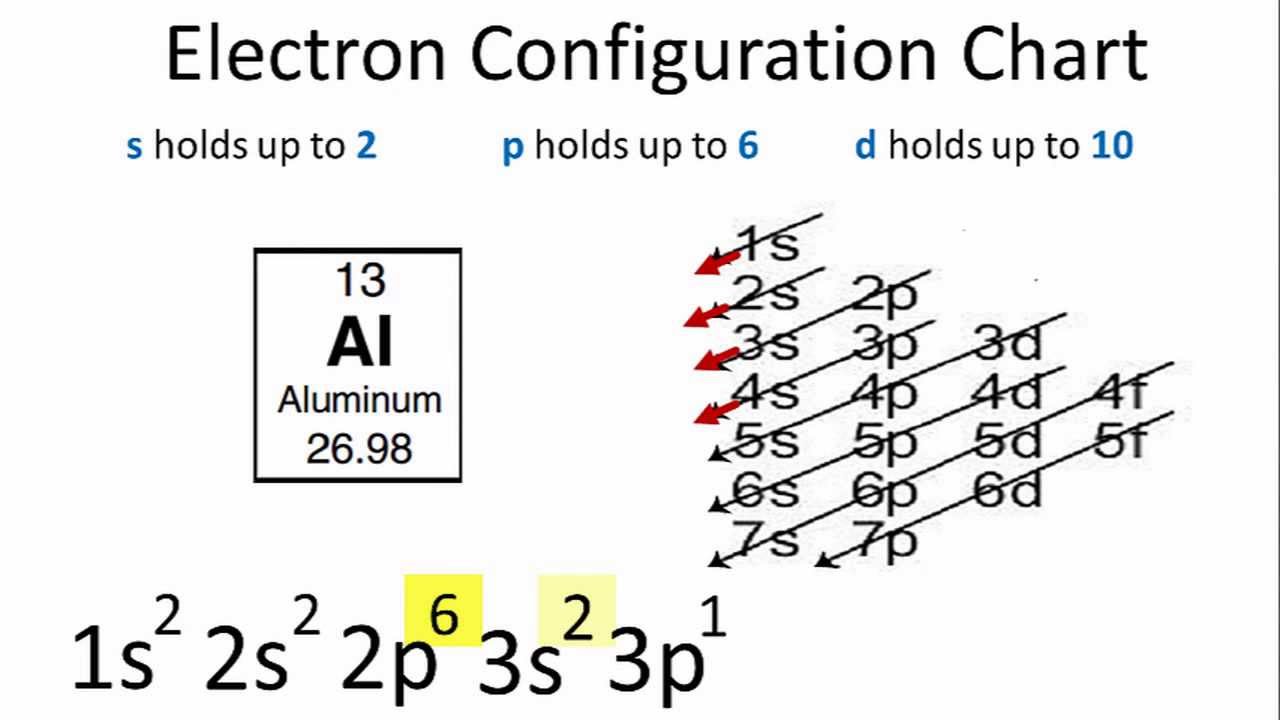 Electron Configuration For Aluminium Al
Electron Configuration For Aluminium Al
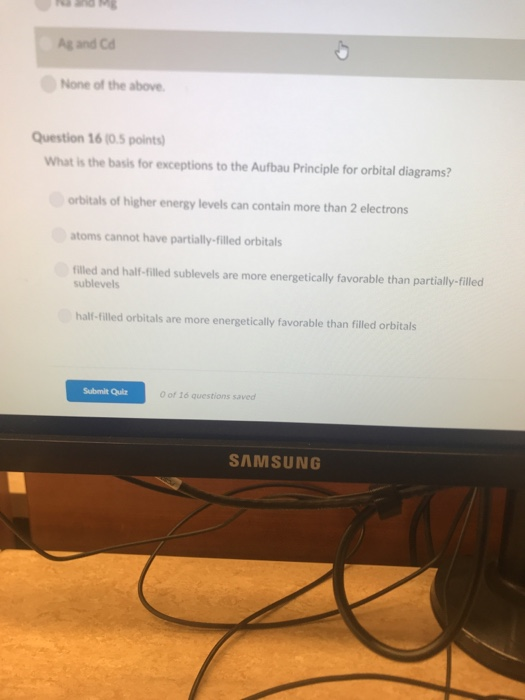 Solved False Question 6 0 5 Point How Many Unpaired Elec
Solved False Question 6 0 5 Point How Many Unpaired Elec
Strong Chemical Bonds Notario Major Reference Works
 Exceptions To Electron Configuration Concept
Exceptions To Electron Configuration Concept
:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png) Electronic Structure And The Aufbau Principle
Electronic Structure And The Aufbau Principle
 Quantum Numbers And Electron Configurations
Quantum Numbers And Electron Configurations
0 Response to "What Is The Basis For Exceptions To The Aufbau Diagram"
Post a Comment