Degrees Of Freedom Phase Diagram
And the composition of the vapor phase is given by where that same line intersects the upper curve. Note the critical point on a phase diagram can only exist at one temperature and pressure for a substance or system and thus the degrees of freedom at any critical point is zero.
Lever Rule Applied To Phase Diagram For Partially Miscible
If there is only one component there are no degrees of freedom f 0 when there are three phases.
Degrees of freedom phase diagram. Totals for both phases since. This is called a phase diagram and when many phases are available there will be many curves in the parameter space to distinguish which phase will be exhibited at those parameters values. Phase diagram and degrees of freedom a phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases.
A system with one component and one phase a balloon full of carbon dioxide perhaps has two degrees of freedom. Temperature as the single degree of freedom. F n m 2.
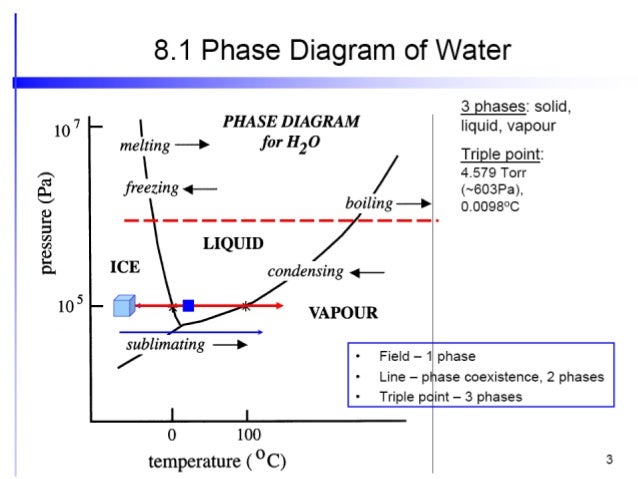
The degrees of freedom of a system dictate the number of phases as described above in the bullet list that can occur in the system. D f p binary solid liquid at constant t p. Therefore in a single component system this three phase mixture can only exist at a single temperature and pressure which is known as a triple point.
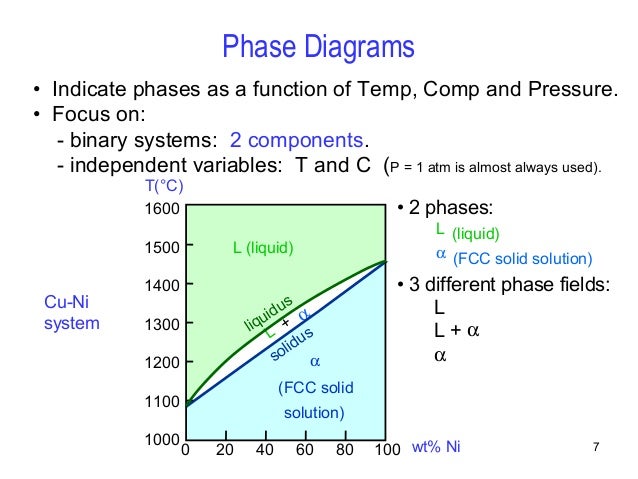
Or to show what phases are present in the material system at various t p and compositions. The composition of the liquid phase is given by where that horizontal line for intersects the lower curve on your chart. Temperature and pressure say can be varied independently.
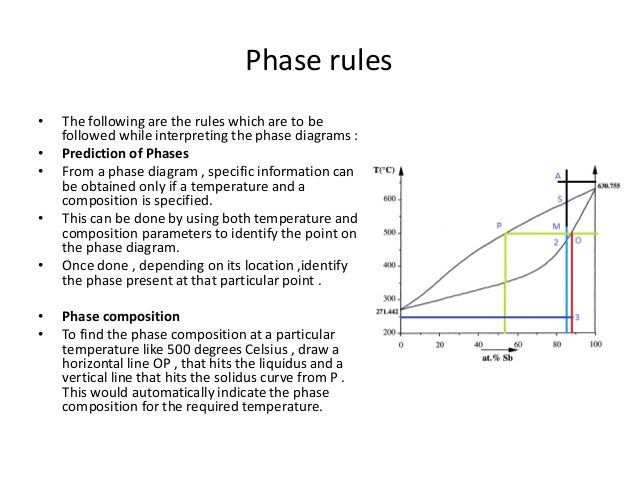
That is there is a function t p that gives the temperature at which water will boil at a particular ambient pressure. µas µal and µbs µbl doesnt matter which phase a b liquid solid a solid b p cst 0 1 xa. The gibbs phase rule describes the possible number of degrees of freedom f in a closed system at equilibrium in terms of the maximum number of stable phases m and the number of system components n.
If you have two phases liquid and vapour for instance you lose a degree of freedom and there is only one possible pressure for each temperature. F 2 2 0 d f p 0 2 2 dg µa dn a µb dn b dn a and dn b. Thus now that weve specified temperature.
 Figure 11 From 2 Phase Diagram Analysis For Predicting
Figure 11 From 2 Phase Diagram Analysis For Predicting
Solid Solutions And Phase Equilibrium
Excited State Quantum Phase Transitions In Systems With Two
 Phase Rule An Overview Sciencedirect Topics
Phase Rule An Overview Sciencedirect Topics
 Chapter 6 Phase Equilibria Ppt Video Online Download
Chapter 6 Phase Equilibria Ppt Video Online Download
 Hot Under The Collar Part Iii Phase Diagrams Ppt Video
Hot Under The Collar Part Iii Phase Diagrams Ppt Video
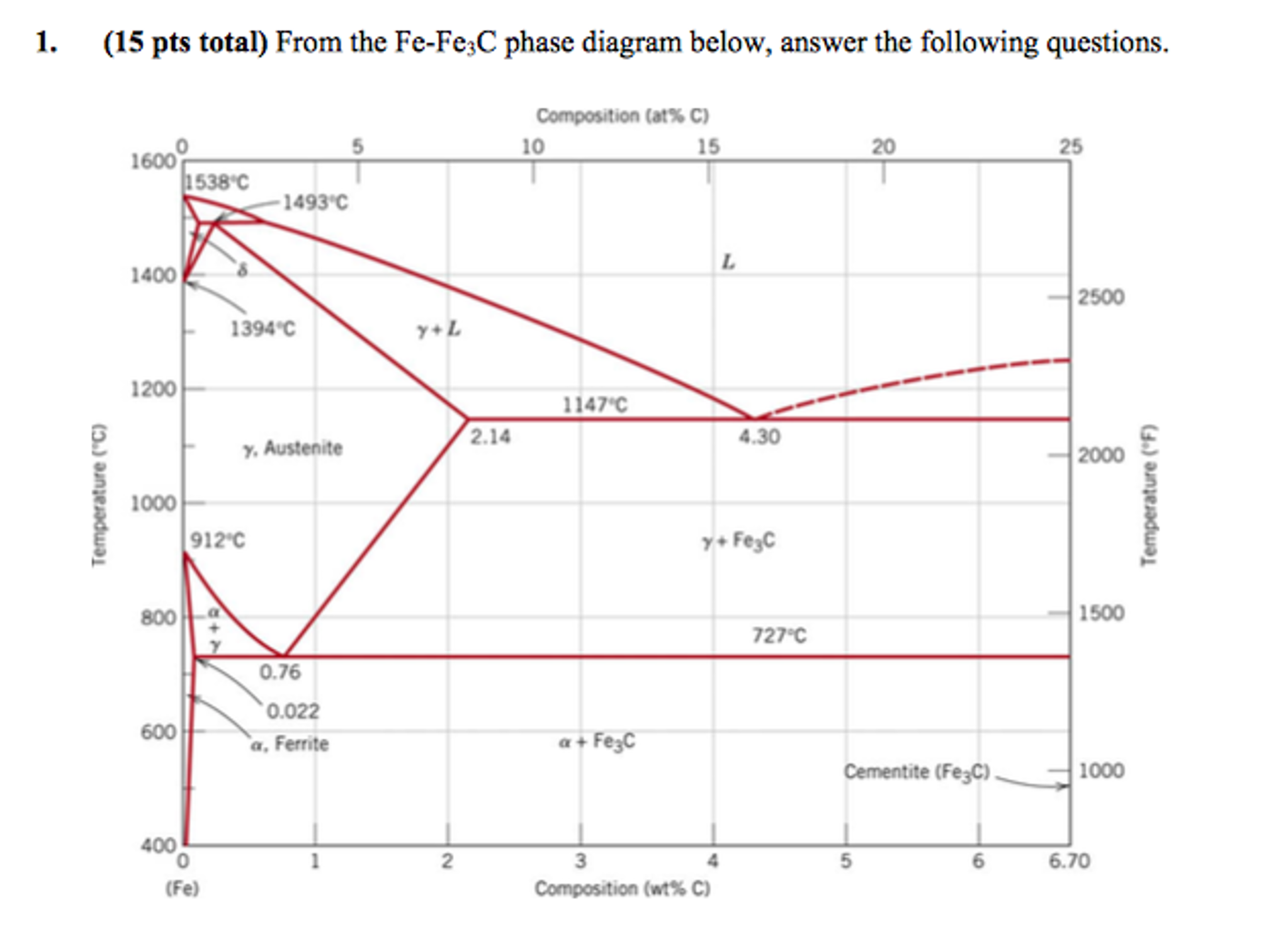 From The Fe Fe 3c Phase Diagram Below Answer The
From The Fe Fe 3c Phase Diagram Below Answer The
 Gibbs Phase Rule Phase Diagrams Degrees Of Freedom
Gibbs Phase Rule Phase Diagrams Degrees Of Freedom
 Phase Diagrams Book Chapter Iopscience
Phase Diagrams Book Chapter Iopscience
 Critical Point Thermodynamics Wikipedia
Critical Point Thermodynamics Wikipedia
 Solved In Figure Is Shown The Pressure Temperature Phase
Solved In Figure Is Shown The Pressure Temperature Phase
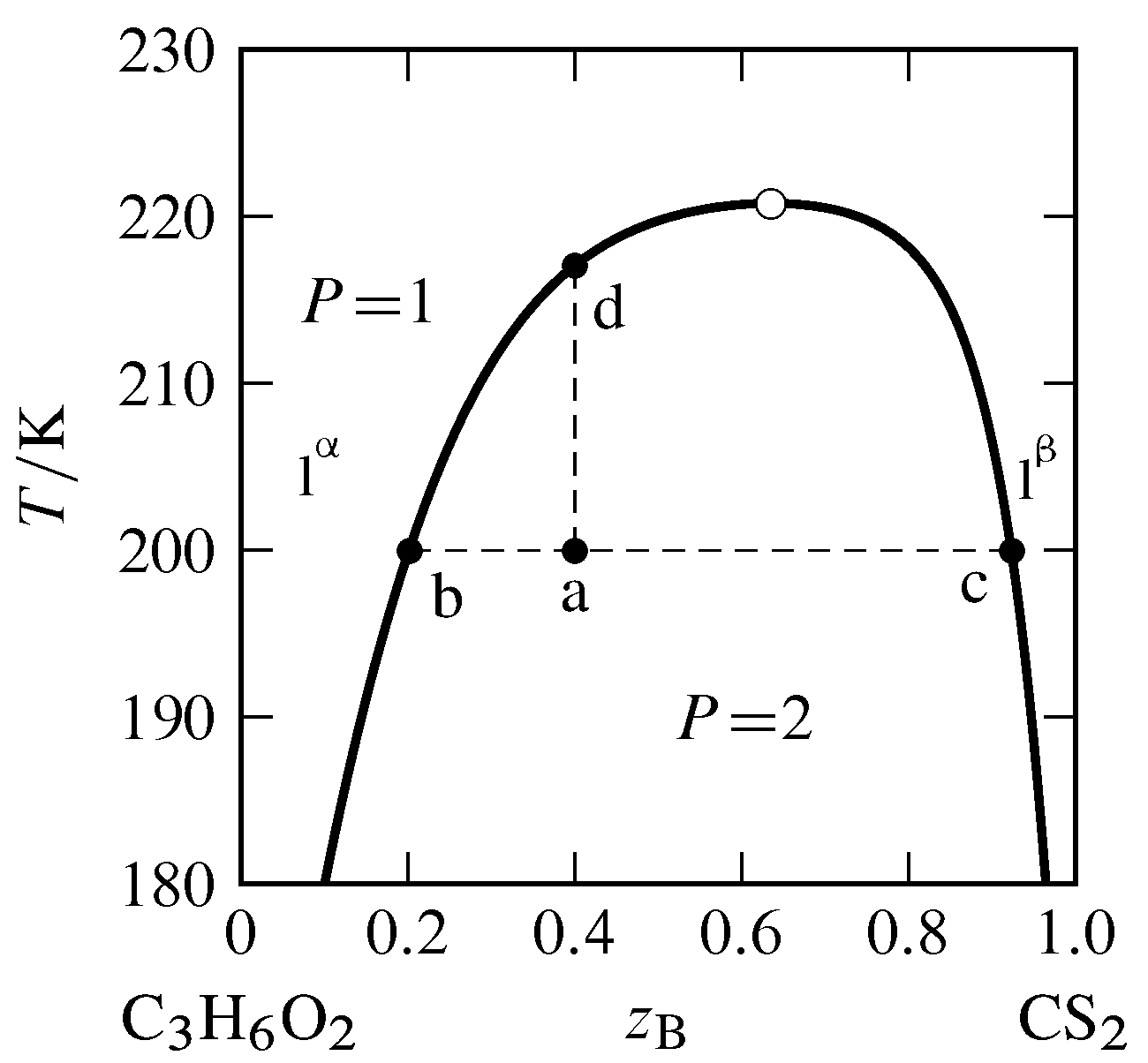 13 2 Phase Diagrams Binary Systems Chemistry Libretexts
13 2 Phase Diagrams Binary Systems Chemistry Libretexts
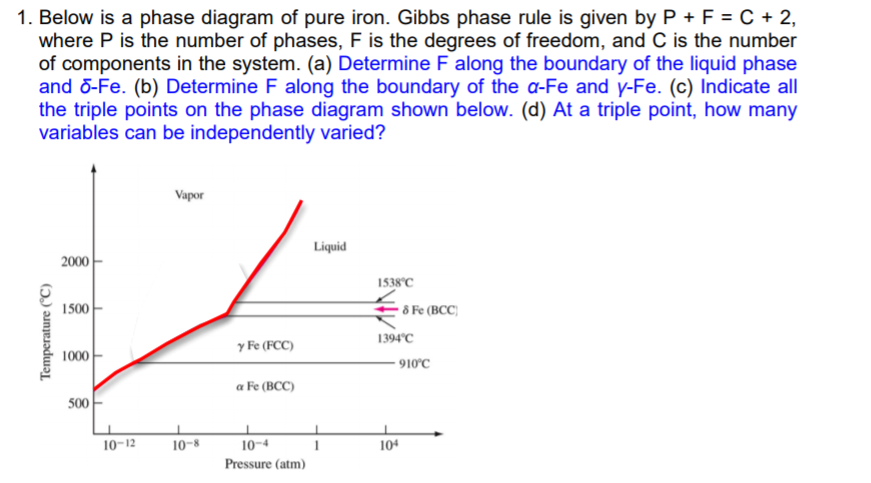 Solved 1 Below Is A Phase Diagram Of Pure Iron Gibbs Ph
Solved 1 Below Is A Phase Diagram Of Pure Iron Gibbs Ph
Metamorphic Petrology Geology 102c
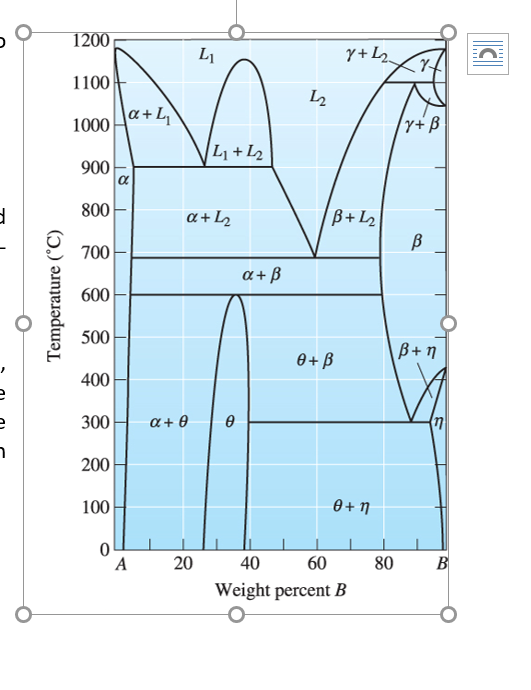 Solved Use The Gibbs Phase Rule To Verify That A Three Ph
Solved Use The Gibbs Phase Rule To Verify That A Three Ph
 The Low Temperature H Vs T Phase Diagram For Ceb 6 Displays
The Low Temperature H Vs T Phase Diagram For Ceb 6 Displays
 Phase Rule An Overview Sciencedirect Topics
Phase Rule An Overview Sciencedirect Topics






0 Response to "Degrees Of Freedom Phase Diagram"
Post a Comment