Which Of The Following Diagrams Represent A Weak Acid Dissolved In Water
A rank the acids in order of increasing k a. Which of the following nonmetal oxides produce a weak acid when dissolved in water.
 Solution Hypochlorous Acid Hclo Is A We Clutch Prep
Solution Hypochlorous Acid Hclo Is A We Clutch Prep
B rank the acids in order of increasing p k a.
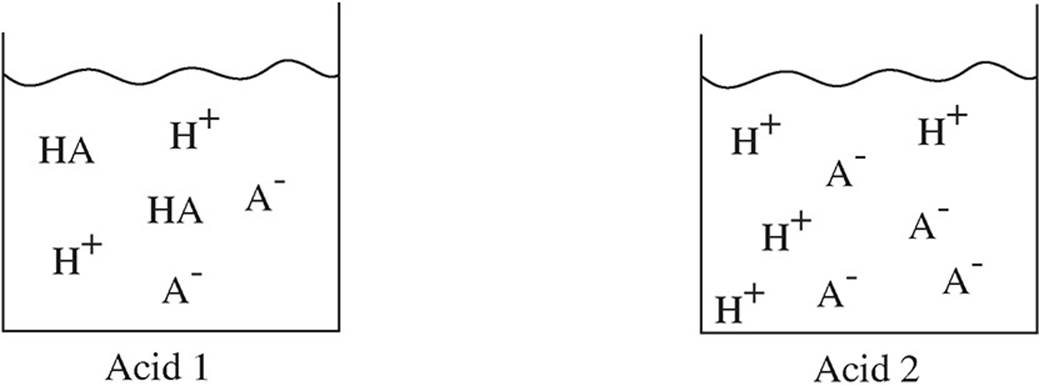
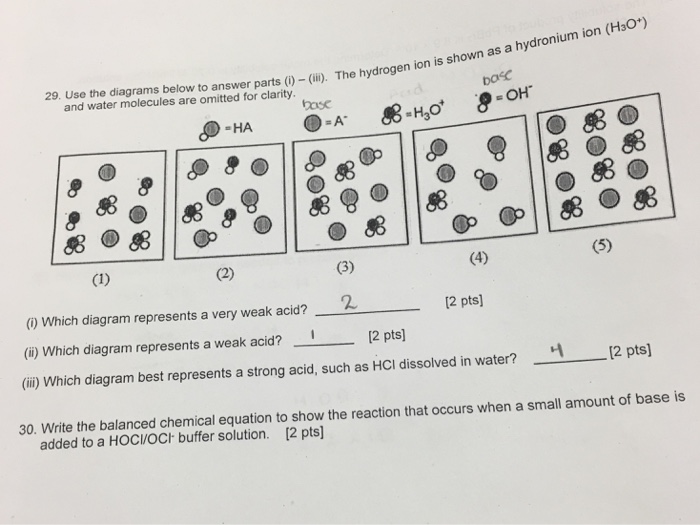
Which of the following diagrams represent a weak acid dissolved in water. Which represents a very weak acid. Which of the following diagrams best represents a strong acid such as hcl dissolved in water. The hydrated proton is shown as a hydronium ion.
As given all acids are monoprotic that means each unit of the acid yields one ion upon ionization. An acid is defined as a substance that yields hydrogen ions when dissolved in waterif an acid gives more number of hydrogen ions when dissolved in water is called strong acidan acid that gives less number of hydrogen ions is called weak acid. Example 2 ph calculations for weak acid solutions.
Which represents a very weak acid. The reaction between the ion of a weak acid or a weak base and water is called aan reaction. Vinegar is a dilute water solution of acetic acid with small amounts of other components.
The following scenes represent three weak acids ha where a x y or z dissolved in water 2 o is not shown. A h2o b kcl c hno3 d hc2h3o2 e c12h22o11. Water molecules are omitted for clarity a b c d.
Kb hb oh b. The following pictures represent solutions at various points in the titration of a weak acid ha with aqueous koh. Which represents a weak acid.
K h3o initially present oh initially present and solvent water molecules have been omitted for clarity. Which of the following correctly represents kb for a weak base of general formula b. Which of the following diagrams best represents a strong acid such as hcl dissolved in water.
Which represents a weak acid. Water molecules are omitted for clarity. The hydrated proton is shown as a hydronium ion.
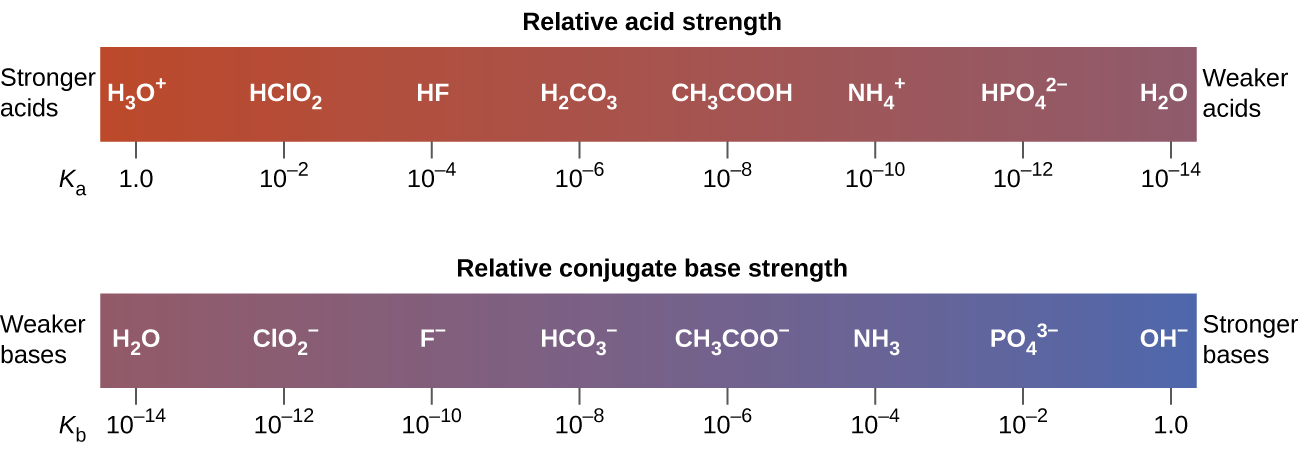
C rank the conjugate bases in order of increasing p k b. Unshaded spheres represent h atoms black spheres represent oxygen atoms and shaded spheres represent a ions. Co₂ cl₂o₇ p₄o₁₀ so₃ n₂o₅.
The diagram best represent the hydration is c because nacl ions are separated and h positive inos is attracted to cl which are negative. D what is the percent dissociation of hx. Calculate the ph of bottled vinegar that is 0667 m hc 2 h 3 o 2 assuming that none of the other components affect the acidity of the solution.
3identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte.
Chapter 11 Acids And Bases Practice Problems Section

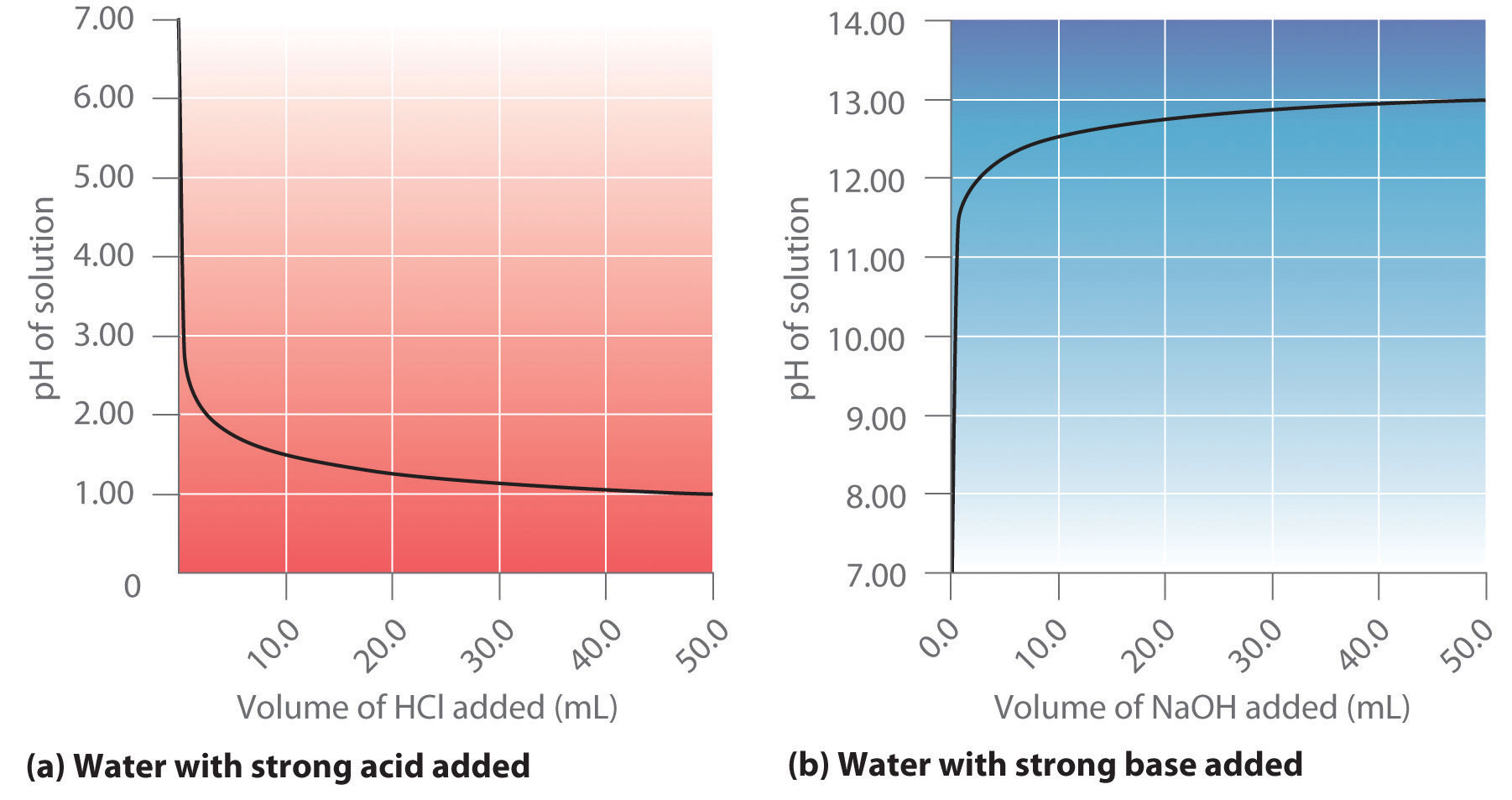
Water Ionization The Ionic Product Kw Of Water And Ph

 16 6 Weak Acids Chemistry Libretexts
16 6 Weak Acids Chemistry Libretexts
 Practice Test 1 Practice Tests Cracking The Ap Chemistry
Practice Test 1 Practice Tests Cracking The Ap Chemistry
Chapter 16 Acids Bases And Salts
 Percent Ionization For A Weak Acid Ha Is Determined By The Following Formula Percent Ionization Ha Ionized Ha Initial 100 For Strong Acids Ionization Is Nearly Complete 100 At Most
Percent Ionization For A Weak Acid Ha Is Determined By The Following Formula Percent Ionization Ha Ionized Ha Initial 100 For Strong Acids Ionization Is Nearly Complete 100 At Most
 Acids Water Diagram Wiring Diagram
Acids Water Diagram Wiring Diagram
 Chemistry Ii Water And Organic Molecules
Chemistry Ii Water And Organic Molecules
 Solved Which Of The Following Diagrams Best Represents A
Solved Which Of The Following Diagrams Best Represents A
 Chemistry Ii Water And Organic Molecules
Chemistry Ii Water And Organic Molecules
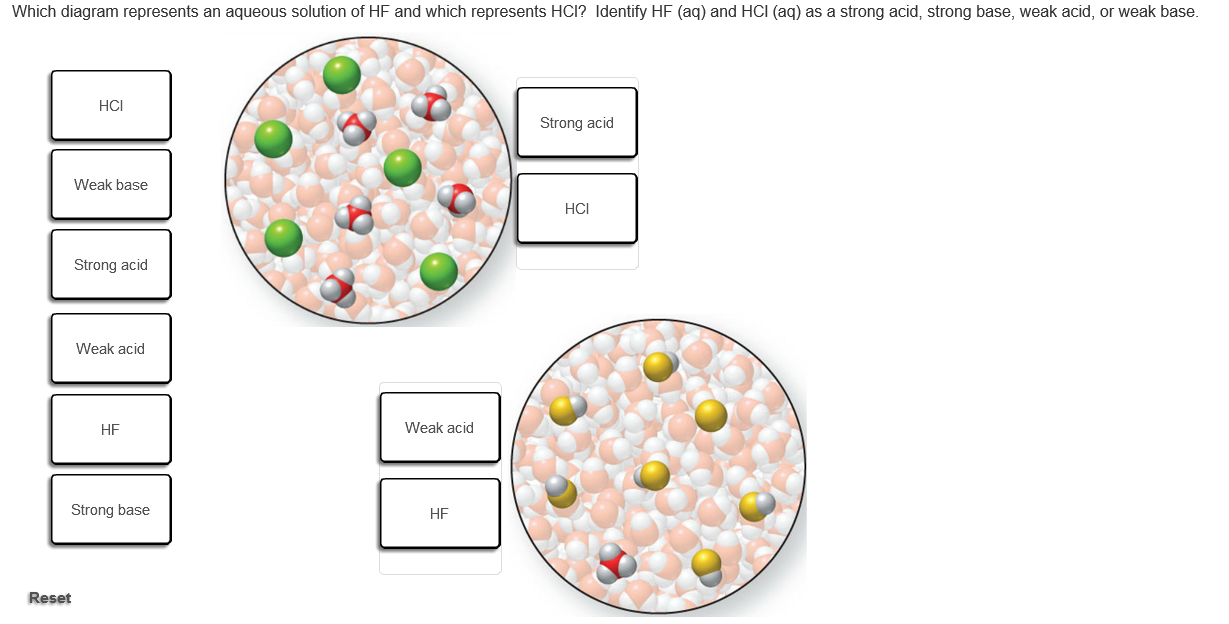 Diagram Acid Solution Wiring Diagrams 24
Diagram Acid Solution Wiring Diagrams 24

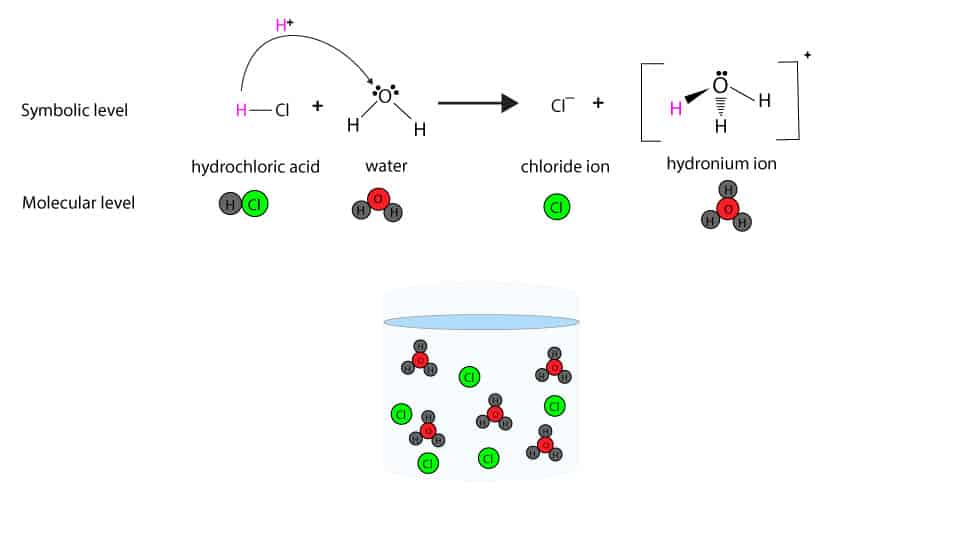
 Hydrogen Chloride Vs Hydrochloric Acid Video Lesson
Hydrogen Chloride Vs Hydrochloric Acid Video Lesson
Water Ionization The Ionic Product Kw Of Water And Ph
 Acid Base Equilibria Varieties Of Citrus Fruits Ranges
Acid Base Equilibria Varieties Of Citrus Fruits Ranges
Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
 Solved Which Of The Following Diagrams Best Represents A
Solved Which Of The Following Diagrams Best Represents A
 What Are Electrolytes In Chemistry Strong Weak And Non
What Are Electrolytes In Chemistry Strong Weak And Non
11 3 Finding The Ph Of Weak Acids Bases And Salts
 H2so4 H2o Sulfuric Acid Plus Water
H2so4 H2o Sulfuric Acid Plus Water
 Solved Which Of The Following Diagrams Best Represents A
Solved Which Of The Following Diagrams Best Represents A
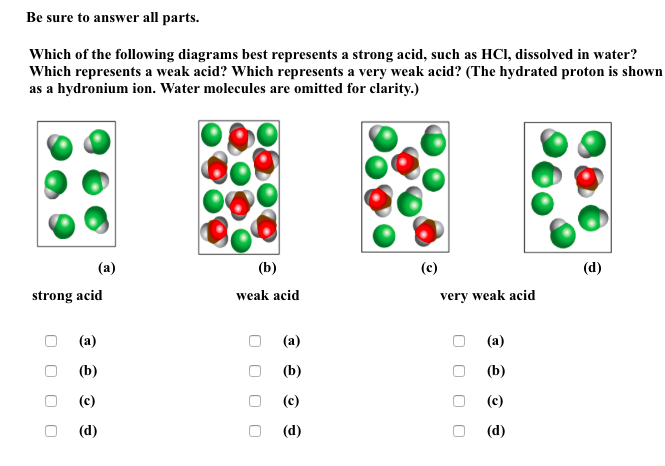 Solved Be Sure To Answer All Parts Which Of The Followin
Solved Be Sure To Answer All Parts Which Of The Followin
0 Response to "Which Of The Following Diagrams Represent A Weak Acid Dissolved In Water"
Post a Comment