One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To
One of the main assumptions of the kinetic molecular theory. Increase the temperature of the water h.
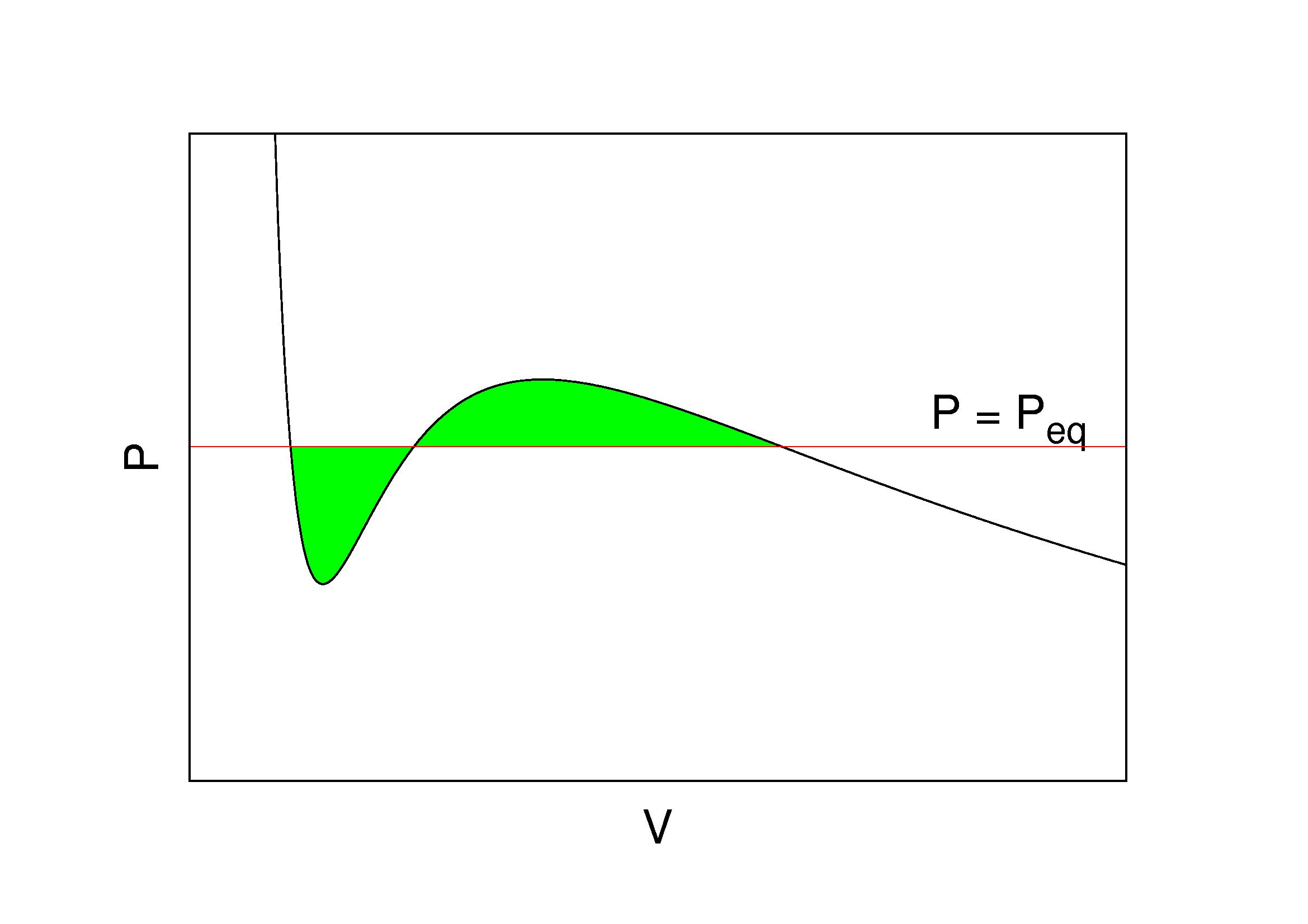 Maxwell Construction Wikipedia
Maxwell Construction Wikipedia
If the volume of gas in the balloon remains constant then an increase in temperature would result in an increased gas pressure in a balloon.

One way to increase the volume of the gas in the balloon in the diagram above is to. Push the balloon farther down into the. Seal the top of the water bath 6. Increase the temperature of the water c.
Triple the density triple the pressure. This is the v in the equation. Three ways to increase the pressure of a gas.
1 increase in volume thus decrease in pressure gas doing work on something thus loses internal energy according to 1st law thermo adiabatic insulated. Where p is the pressure of a gas v is its volume n is the number of moles of the gas t is its temperature on the kelvin scale and r is a constant called the ideal gas constant or the universal gas constant. One of the main assumptions of the.
So pressure is proportional to density. Decrease the volume of the gas. Increase the temperature of the gas.
Gas in the balloon in the diagram. Cool the gas and the balloon only b. Increasing temperature adds energy to the gas molecules increasing their motion and again increasing collisions.
That result can be achieved in three ways. The density of air can also be doubled by simply compressing the air to half its volume. This is represented by t in the equation.
One way to increase the volume of the. Gas expands decrease in internal energy since no heat transfer in thus loss of ke pressure and temperature increase 2 temperature will increase because temperature and volume proportional. Kinetic molecular theory of gases is.
How could he now study the effects of. One way to increase the volume of the gas in the balloon in the diagram to the left is to a. Seal the top of the water bath 6.
Experiment to determine how one variable affects the other. That the particles of an ideal gas. And to analyze a.
Push the balloon farther down into the water bath d. 3 trial volume pressure temperature 1 100 ml 250 mm hg 298 k 2 300 ml 83 mm hg 298 k 3 500 ml 50 mm hg 298 k a student wants to study the effects of volume on gas pressureduring his experiment he recorded the above data. Push the balloon farther down into the water bath d.
Above is to f. One way to increase the volume of the gas in the balloon in the diagram below is to a. Combining these four laws yields the ideal gas law a relation between the pressure volume temperature and number of moles of a gas.
Increase the temperature of the water c. Cool the gas in the balloon only. We increase the density of a balloon when we squeeze it and likewise increase air dentiy in the cylinder of a tire pump when we push the piston downward.
Seal the top of the water bath. Cool the gas and the balloon only b.
 Wait Weight Don T Tell Me Chemistry Earth Science
Wait Weight Don T Tell Me Chemistry Earth Science
 Motion And Forces Newton S Third Law Of Motion Perkins
Motion And Forces Newton S Third Law Of Motion Perkins
Ideal Gas Law Forces That Cause Air To Rise Or Sink
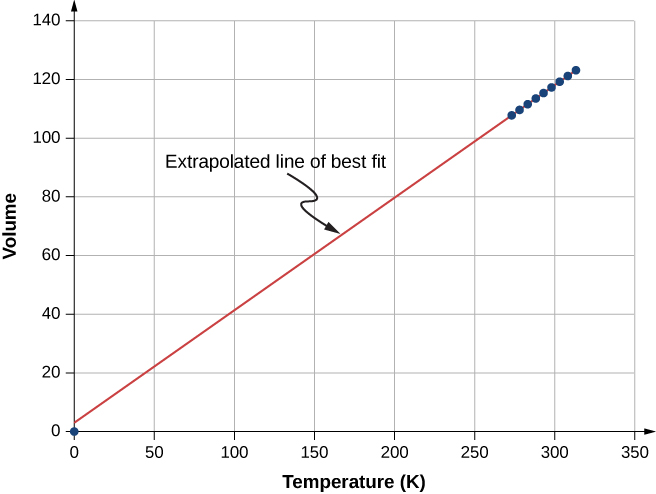 2 1 Molecular Model Of An Ideal Gas Physics Libretexts
2 1 Molecular Model Of An Ideal Gas Physics Libretexts
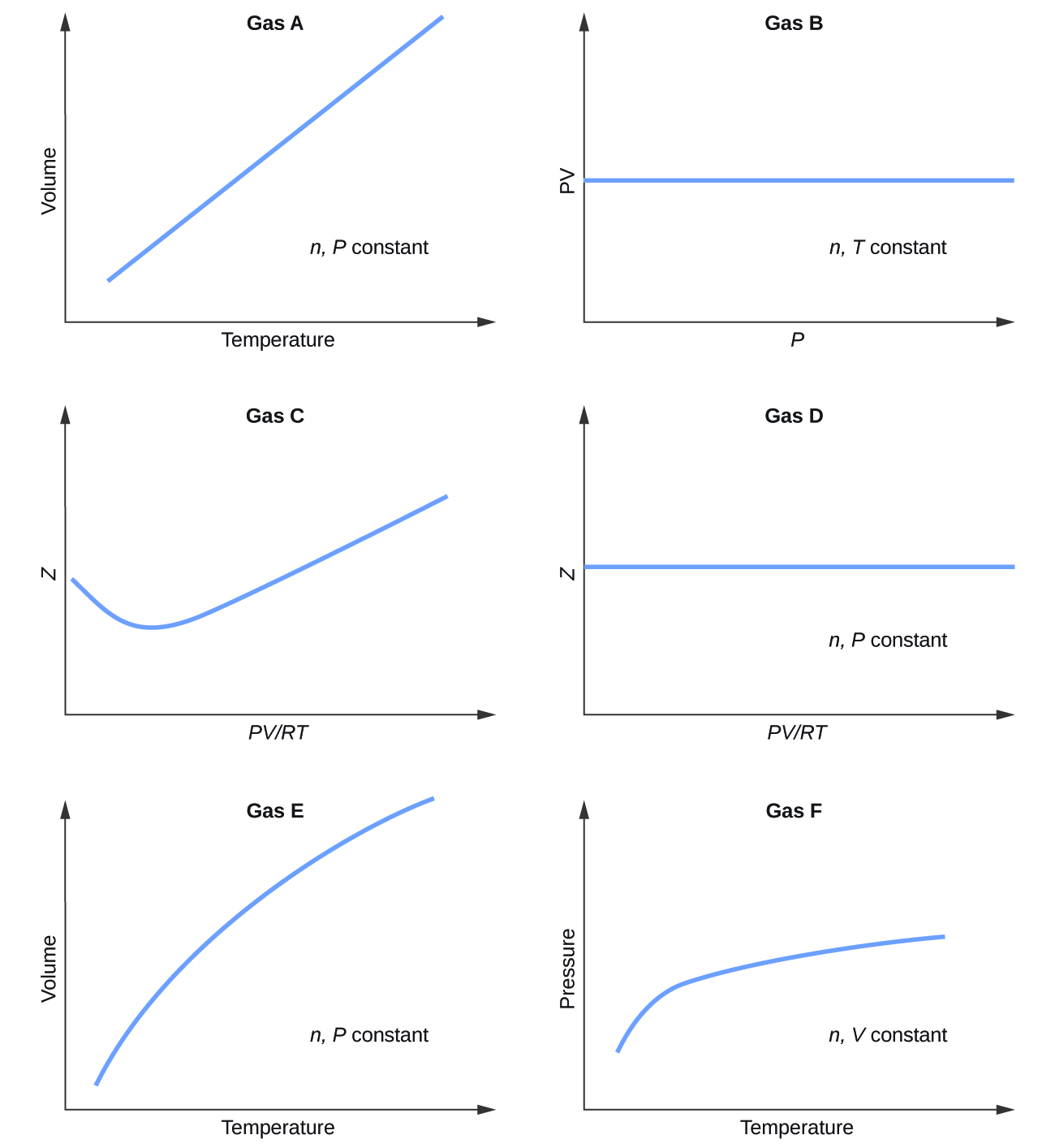 9 E Gases Exercises Chemistry Libretexts
9 E Gases Exercises Chemistry Libretexts
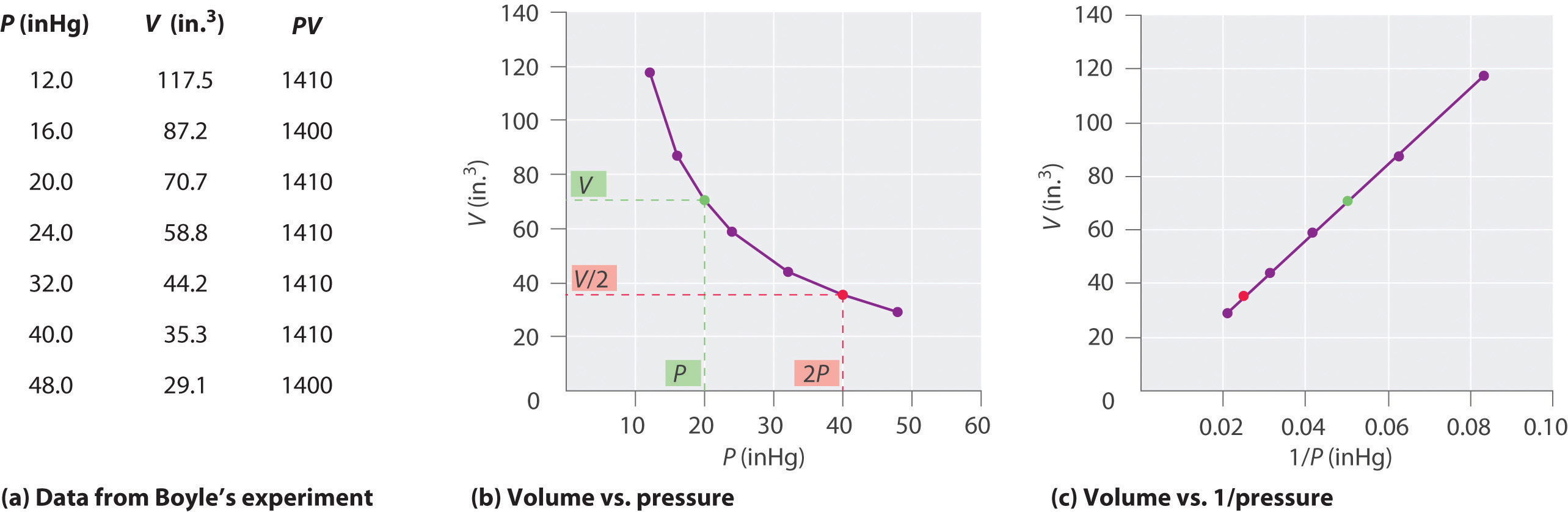 6 3 Relationships Among Pressure Temperature Volume And
6 3 Relationships Among Pressure Temperature Volume And
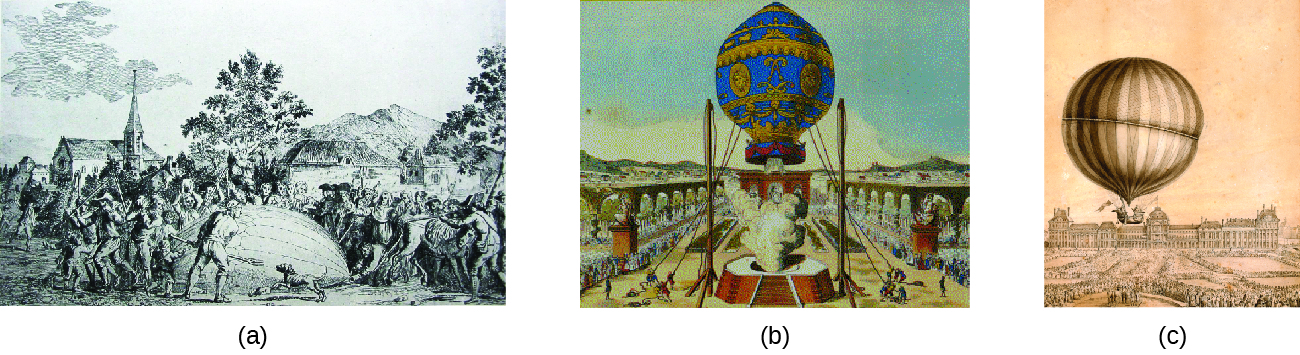 9 2 Relating Pressure Volume Amount And Temperature The
9 2 Relating Pressure Volume Amount And Temperature The
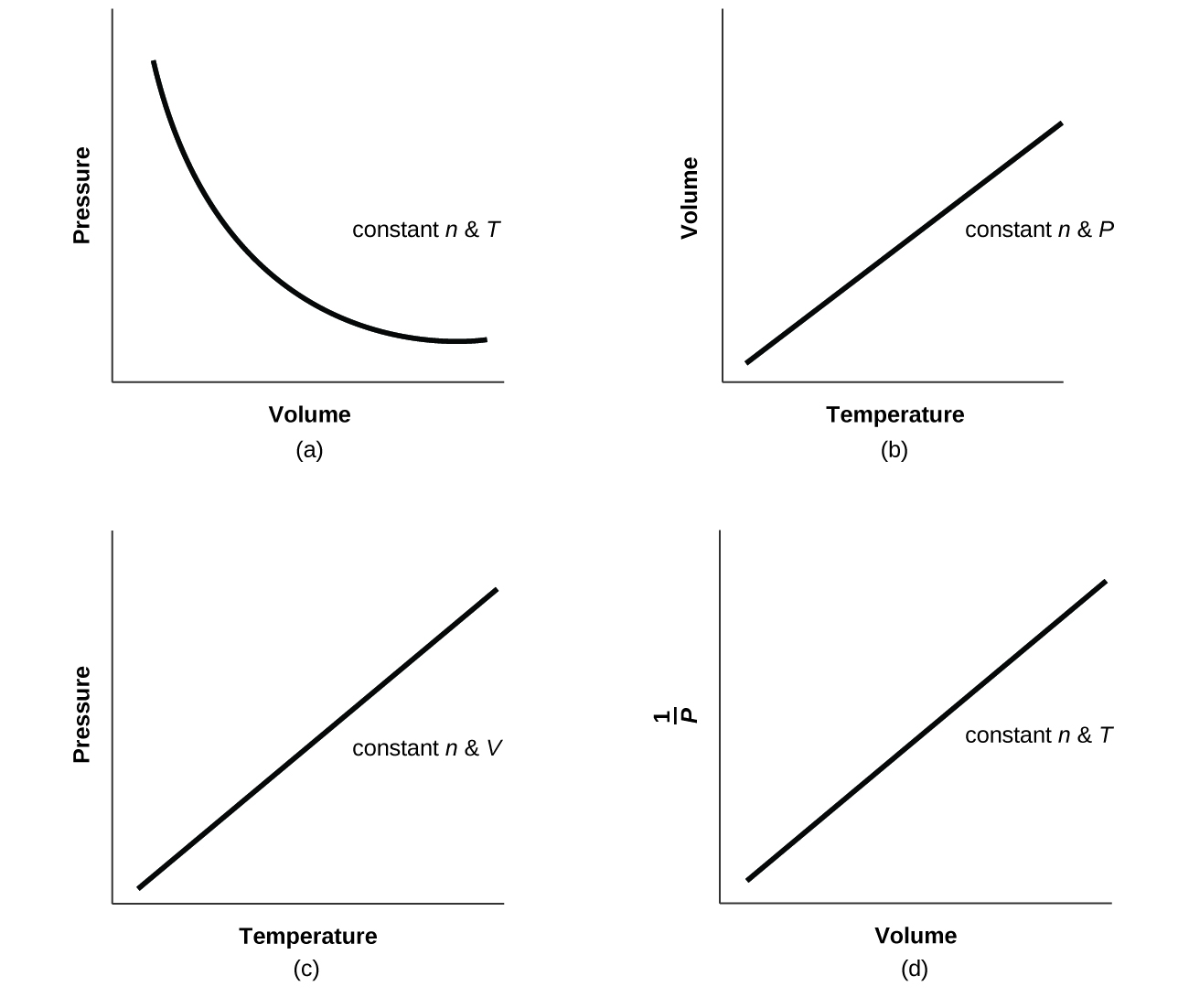 9 2 Relating Pressure Volume Amount And Temperature The
9 2 Relating Pressure Volume Amount And Temperature The
 Is The Density Of The Air In A Heated Hot Air Balloon Less
Is The Density Of The Air In A Heated Hot Air Balloon Less
Ideal Gas Law Forces That Cause Air To Rise Or Sink
Gases Liquids Solids States Of Matter Kinetic Particle
Ideal Gas Law Forces That Cause Air To Rise Or Sink
 How Ventilation Muscles Cause Inspiration And Expiration
How Ventilation Muscles Cause Inspiration And Expiration
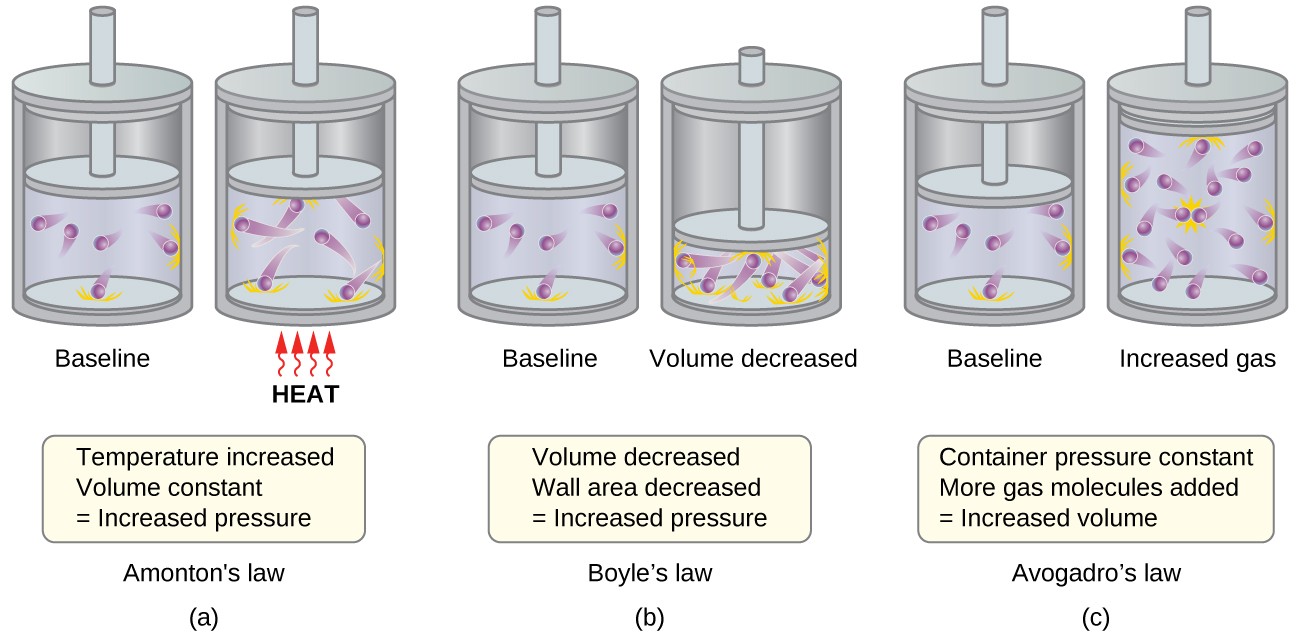 The Kinetic Molecular Theory Chemistry
The Kinetic Molecular Theory Chemistry
Particle Model Latent Heat Internal Energy State Changes Gas
 Gas Laws Why Do Helium Balloons Expand In Volume As They
Gas Laws Why Do Helium Balloons Expand In Volume As They





0 Response to "One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To"
Post a Comment