How Is Activation Energy Represented On An Energy Diagram
Aactivation energy is the same for both endothermic and exothermic reactions. D is the energy of the products.
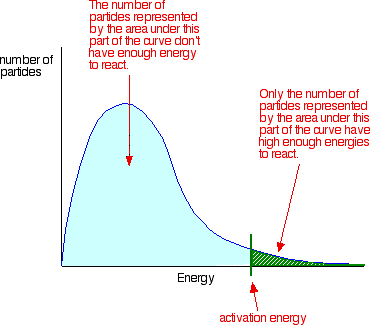 The Effect Of Catalysts On Rates Of Reaction
The Effect Of Catalysts On Rates Of Reaction
What is the activation energy for the reverse reaction.
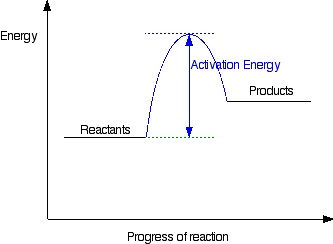
How is activation energy represented on an energy diagram. Can i have an explanation please. This first video takes you through all the basic parts of the pe diagram. 2when dissolved in 1000 g of water which chemical compound will produce a.
The reaction is exothermic because the energy of the reactants is greater than the energy of the products. A is the activation energy. The activation energy diagram is drawn as a hill because there is a large amount of energy needed to form the unstable transition state viewed as the upward slope on the diagram.
Activation energy and reverse reactions. How is activation energy represented on an energy diagram. Not all reactions are reversible.
The activation energy for the following reaction is 125 kjmol no2co no co2 the change in energy delta e is 216 kjmol. Activation energy is the energy that molecules have to possess for a reaction to occur. Dactivation energy is represented by a negative slope.
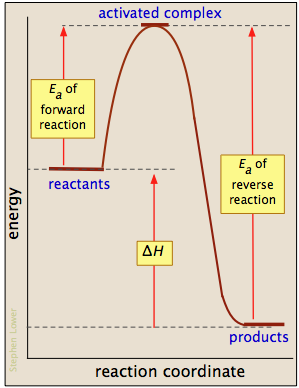
The chemical equation is going to represent energy written with the products or subtracted from the reactants endothermic potential energy diagram a chemical reaction where the potential energy of the products is higher than that of the reactants. Cactivation energy is greater for exothermic reactions. B is the activated complex.
1on a potential energy diagram what is true about activation energy. E is the energy of the reactants. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction.
Bactivation energy is greater for endothermic reactions. F is the reactants q r. It is associated with the.
But if we consider a reversible reaction with following hypothetical potential energies potential energy of reactants 200 kj transition state 500 kj products 100 kj the activation energy for the forward rea. The activation energy diagram is drawn as a hill because there is a large amount of energy needed to form the unstable transition state viewed as the upward slope on the diagram. C is the enthalpy of reaction.
Sometimes a teacher finds it necessary to ask questions about pe diagrams that involve actual potential energy values. G is the products n m.
 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
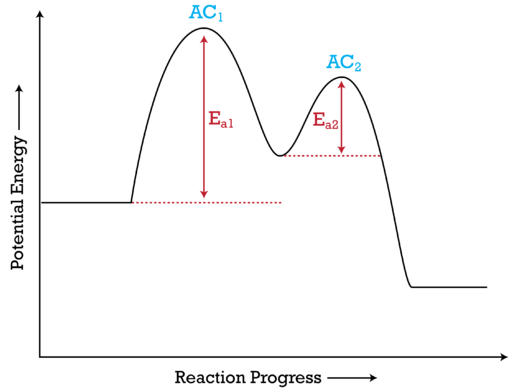 18 15 Mechanisms And Potential Energy Diagrams Chemistry
18 15 Mechanisms And Potential Energy Diagrams Chemistry
Mrs Coon S Chemistry Classroom
 Organic Reactions Regents In One Industrial Organic
Organic Reactions Regents In One Industrial Organic
 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 Reaction Mechanism Britannica Com
Reaction Mechanism Britannica Com
 Which Arrow Represents The Activation Energy Of The Forward
Which Arrow Represents The Activation Energy Of The Forward
 Activation Energy Of Enzymes Definition Calculation
Activation Energy Of Enzymes Definition Calculation
 How Can I Represent The Activation Energy In A Potential
How Can I Represent The Activation Energy In A Potential
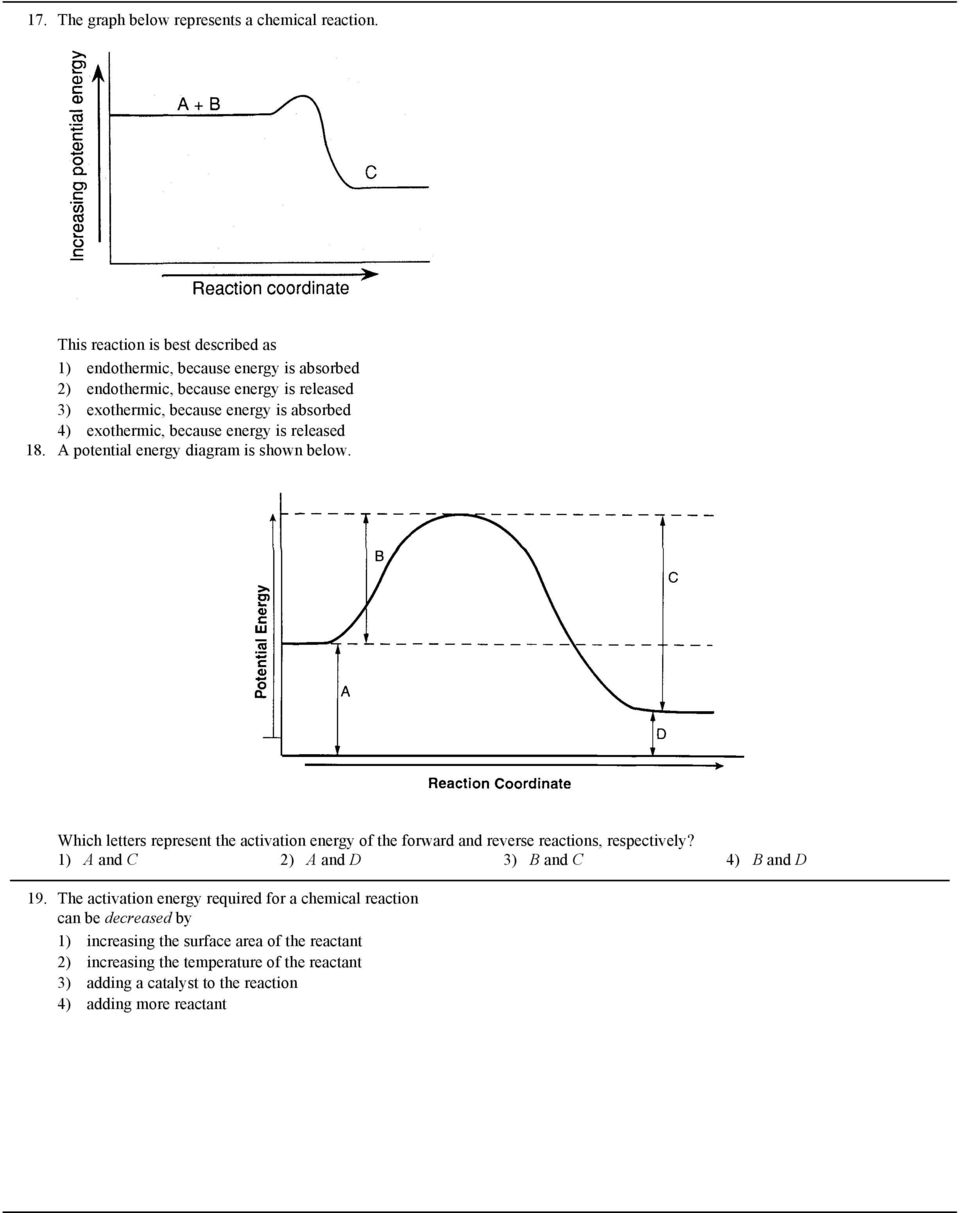 1 The Graph Below Represents The Potential Energy Changes
1 The Graph Below Represents The Potential Energy Changes
 Solved Chapter 4 Problem 35sp Solution Organic Chemistry
Solved Chapter 4 Problem 35sp Solution Organic Chemistry
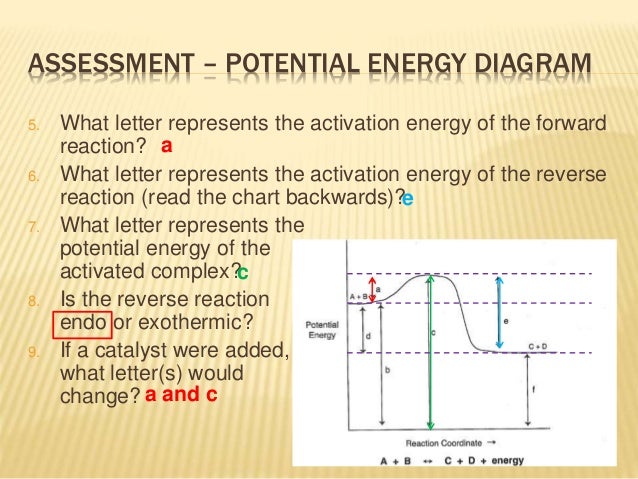 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams
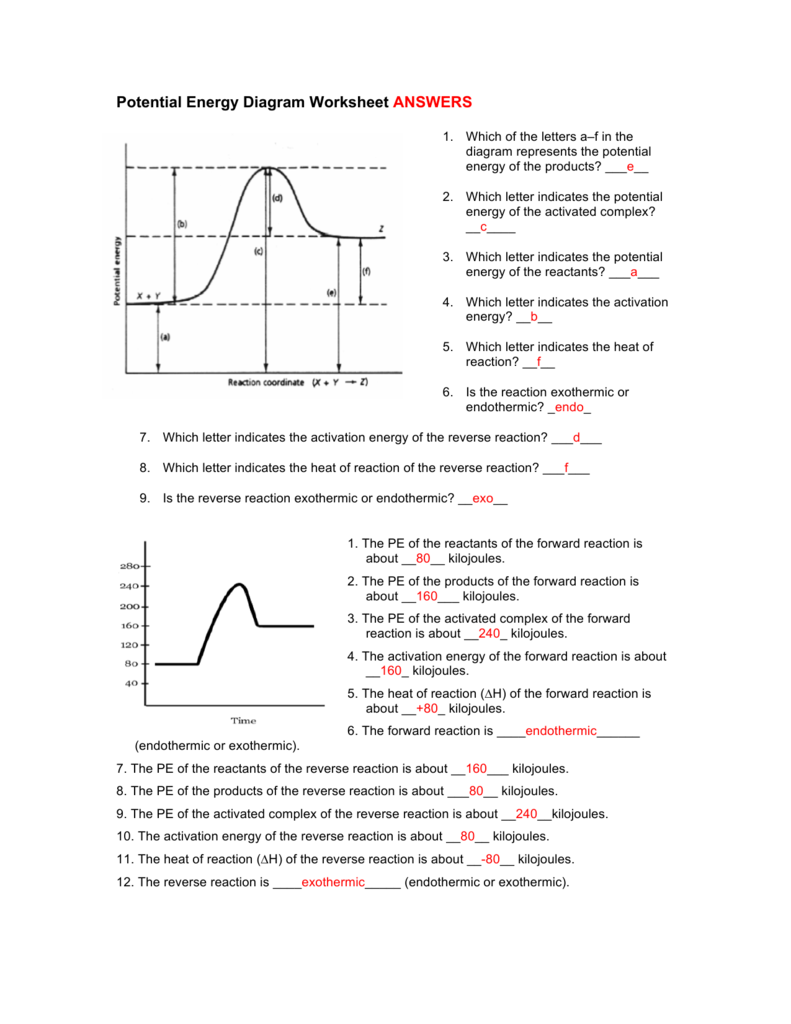 Potential Energy Diagram Worksheet Answers
Potential Energy Diagram Worksheet Answers
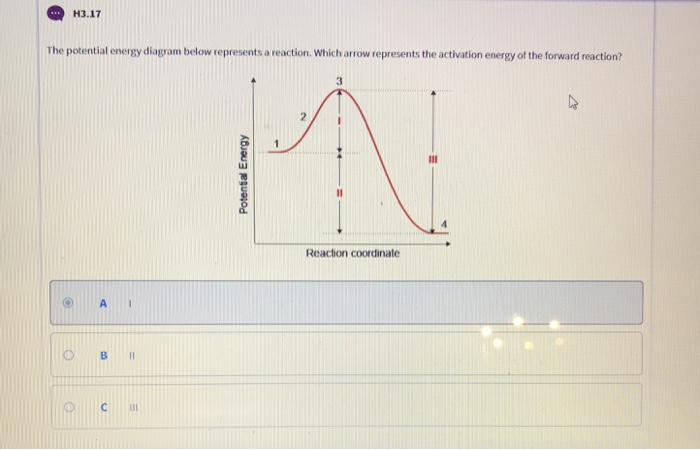 Solved H3 17 The Potential Energy Diagram Below Represent
Solved H3 17 The Potential Energy Diagram Below Represent
Basics Of Reaction Profiles Chemistry Libretexts
 Looking At A Potential Energy Diagram What Is The Name Given
Looking At A Potential Energy Diagram What Is The Name Given
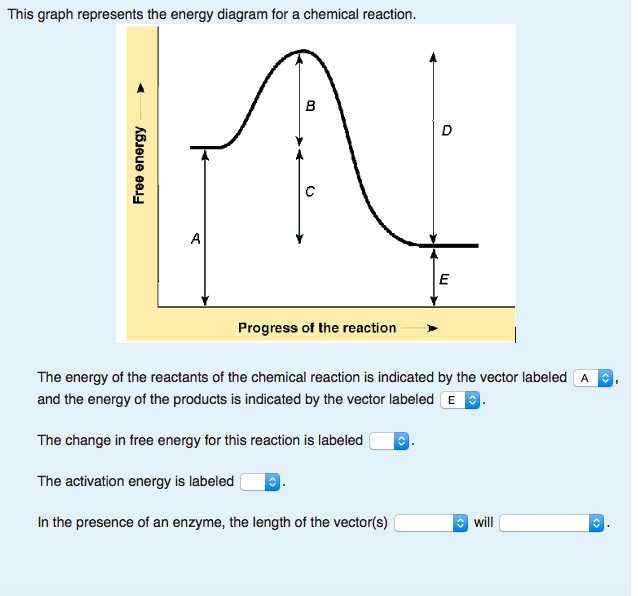 Solved The Graph Represents The Energy Diagram For A Chem
Solved The Graph Represents The Energy Diagram For A Chem
6 1 Activation Energy Kerem S Chemistry Notes Ib
 Represent The Activation Energy Of Both As Prepared And
Represent The Activation Energy Of Both As Prepared And
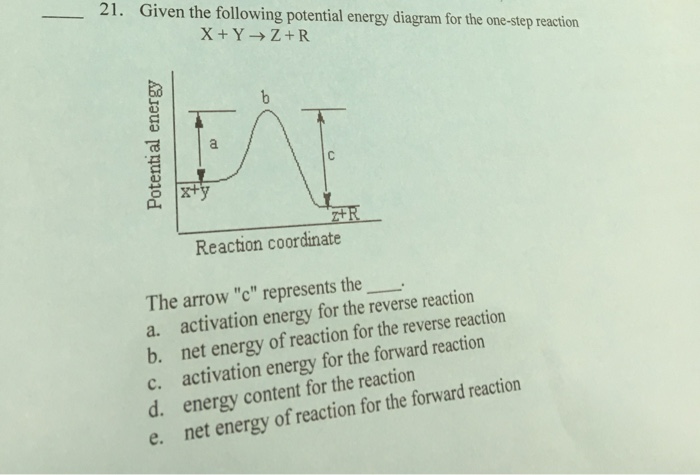
 On The Energy Diagram Below Which Arrows Represent The
On The Energy Diagram Below Which Arrows Represent The
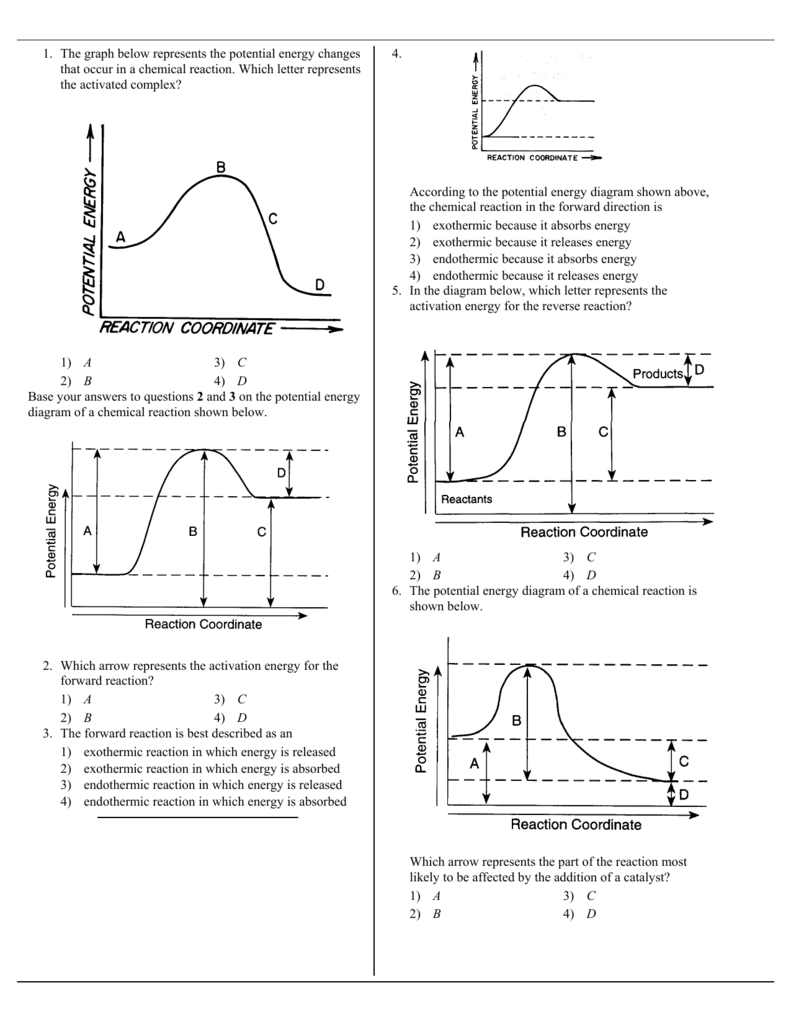 1 The Graph Below Represents The Potential Energy
1 The Graph Below Represents The Potential Energy
Kinetics Of A Reaction Calculating Activation Energy

 Which Reaction Coordinate Diagram Represents A Reaction In Which The Activation Energy Ea Is 50 Kj Mol 1 And The Dhrxn Is 15 Kj Mol 1
Which Reaction Coordinate Diagram Represents A Reaction In Which The Activation Energy Ea Is 50 Kj Mol 1 And The Dhrxn Is 15 Kj Mol 1

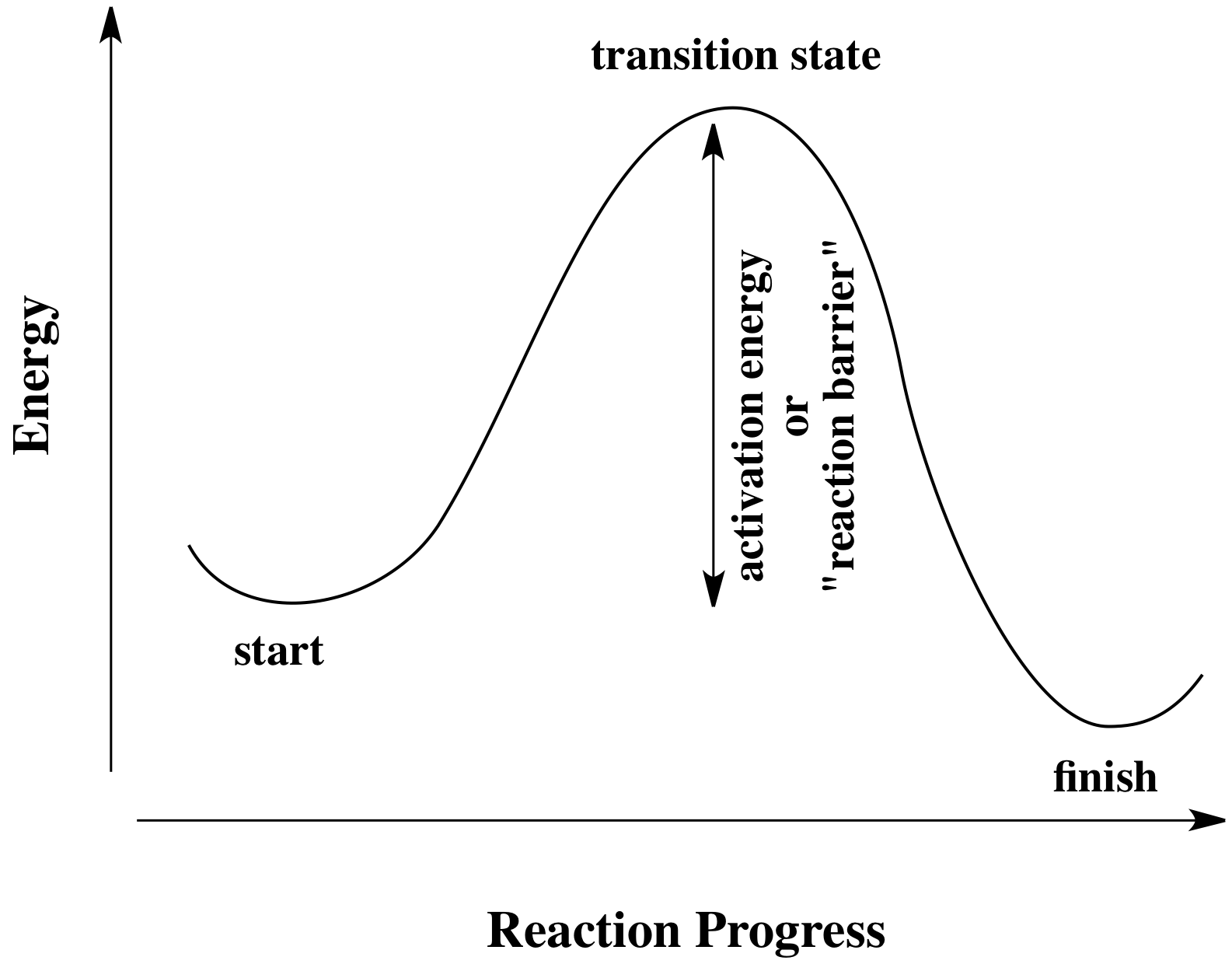

0 Response to "How Is Activation Energy Represented On An Energy Diagram"
Post a Comment